Abstract
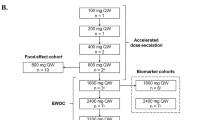
Background: This phase I study was performed to evaluate the safety, tolerability, and efficacy of the oral matrix metalloproteinase inhibitor BAY 12-9566 in combination with doxorubicin in patients with advanced solid tumours, and to identify the maximum tolerated dose of these agents in combination and the dose for use in subsequent studies. Patients and methods: 14 patients were entered onto 3 dose levels consisting of escalating doses of doxorubicin (50 mg/m2, 60 mg/m2 and 70 mg/m2) with 800 mg po bid BAY 12-9566. At all three dose levels, patients received doxorubicin alone in cycle one on day 1. Daily oral dosing with BAY 12-9566 was started on day 8 of cycle 1, and thus doxorubicin was given concurrently with BAY 12-9566 in cycle 2. Patients were continued on treatment until a dose limiting toxicity or tumour progression occurred. Results: Pharmacokinetic studies from cycles 1 and 2 from the patients treated in the first three dose levels demonstrated that the addition of BAY 12-9566 increased the AUC0-12h levels of doxorubicin by a median of 48%. No effects were seen on the BAY 12-9566 pharmacokinetic values. Two dose limiting toxicities were seen at the third dose level. One patient experienced grade 3 stomatitis in cycle 2, and another patient experienced grade 4 granulocytopenia in cycle 1 and grade 4 thrombocytopenia in cycle 2. Thus the maximum tolerated dose of 60 mg/m2 was declared. These toxicities were those that would have been expected from doxorubicin alone. Conclusions: BAY 12-9566 can be safely administered with full doses of doxorubicin without evidence of clinical interaction. The recommended dose of doxorubicin to be combined with BAY 12-9566 800 mg po b.i.d is 60 mg/m2, however, further development of BAY 12-9566 has been abandoned.
Similar content being viewed by others

References
Goetzl E, Banda M, Leppert D: Matrix metalloproteinases in immunity. J Immunol 156: 1–4, 1996
Cawston T: Proteinases and inhibitors. Br Med J 51: 385–401, 1995
De Clerck YA, Shimada H, Taylor SM, Langley KE: Matrix metalloproteinases and their inhibitors in tumor progression. Ann NY Acad Sci 732: 222–232, 1994
Brown PD, Giavazzi R: Matrix metalloproteinase inhibition: a review of anti-tumour activity. Ann Oncol 6: 967–974, 1995
Murphy G, Willenbrock F, Crabbe T, O’Shea M, Ward R, Atkinson S, O’Connell J: Regulation of matrix metalloproteinase activity. Ann NY Acad Sci 732: 31–40, 1994
Levy DE, Ezrin AM: Matrix metalloproteinase inhibiting drugs. Emerging Drugs 2: 205–230, 1997
Kluender H, Dixon B, VanZandt M: Substituted 4-biarylbutyric acid derivatives as matrix metalloproteinase inhibitors. U.S. patent 5,861,428 granted to Bayer Corp. January 19, 1999
Rowinsky E, Humphrey R, Hammond LA, Aylesworth C, Smetzer L, Hidalgo M, Morrow M, Smith L, Garner A, Sorensen JM, Von Hoff DD, Eckhardt SG: Phase I and pharmacologic study of the specific matrix metalloproteinase inhibitor BAY 12–9566 on a protracted oral daily dosing schedule in patients with solid malignancies. J Clin Oncol 18: 178–86, 2000
Grochow L, O’Reilly S, Humphrey R, Sundaresan P, Donehower R, Sartorius S, Kennedy MJ, Armstrong D, Carducci M, Sorensen JM, Kumor K: Phase I and pharmacokinetic study of the matrix metalloproteinase inhibitor (MMPI), BAY 12–9566 (Abstract). Proc Am Soc Clin Oncol 17: 213a, 1998
Erlichman C, Adjei A, Alberts S, Sloan J, Goldberg R, Pitot H, Rubin J: Phase I study of BAY 12-9566—a matrix metalloproteinase inhibitor (MMPI) (Abstract). Proc Am Soc Clin Oncol 17: 217a, 1998
Hirte H, Goel R, Major P, Seymour L, Huan S, Stewart D, Yau J, Arnold A, Holohan S, Waterfield B, Bates S, Bennett K, Walsh W, Elias I: A Phase I dose escalation study of the matrix metalloproteinase inhibitor BAY 12–9566 administered orally in patients with advanced solid tumours. Ann Oncol 11: 1579–1584, 2000
Brundage M, Pater J, Zee B: Assessing the reliability of two toxicity scales: Implications for interpreting toxicity data. J Natl Cancer Inst 85: 1138–1148, 1993
Miller AB, Hoogstraten B, Staquet M, Winkler A: Reporting results of cancer treatment. Cancer 47: 207–214, 1981
Andersen A, Warren DJ, Slordal L: A sensitive and simple high-peformance liquid chromatographic method for the determination of doxorubicin and its metabolites in plasma. Therapeutic Drug Monitoring 15: 455–461, 1993
Agarwal V, Rose D, Krol G: Quantitative HPLC analysis of 4-[4-4-(Chlorophenyl)phenyl]-4-oxo-2S-(phenylthiomethyl)butanoic acid (BAY 12-9566), a metalloproteinase inhibitor and its metabolites in human plasma. Journal of Liquid Chromatography and Related Technologies 22: 1893–106, 1999
Data on file with Bayer Corporation, West Haven, Connecticut
Stewart CF, and Ratain MJ: Topoisomerase Interactive agents. In: Devita VT Jr, Hellman S, and Rosenberg S (eds.), Cancer: Principles and Practice of Oncology 5th edition. Philadelphia: Lippincott-Raven, 1997, pp. 452–467
Steinke W, Gerken F, Chien D-S, Brubaker WF: Whole body autoradiography of BAY 12-9566 in rats after a single intravenous and oral administration (Abstract). Proc Am Assoc Cancer Res 40: 390, 1999
Brubaker WF, Perrino P, Wu Y, Jaworski S, Town C, Jones L, Ward D, Chien D-S: Pharmacokinetics of BAY 12-9566 in animals C species comparison (Abstract). Proc Am Assoc Cancer Res 40: 391, 1999
Tolcher, A, Rowinsky EK, Rizzo J, Britten C, Siu L, Humphrey R, Smetzer L, Sorensen M, Van Hoff DD, Eckhardt SG: A phase I and pharmacokinetic study of the oral matrix metalloproteinase inhibitor BAY 12–9566 in combination with paclitaxel and carboplatin (Abstract). Proc Am Soc Clin Oncol 18: 160a, 1999
Moore MJ, Hamm J, Eisenberg P, Dagenais K, Hagan K, Fields A, Greenberg B, Schwartz B, Ottaway J, Zee B, Seymour L: A comparison between gemcitabine and the matrix metalloproteinase inhibitor BAY 12–9566 in patients with advanced pancreatic cancer (Abstract). Proc Am Soc Clin Oncol 19: 240a, 2000
Grochow LB: Preclinical and clinical pharmacology of matrix metalloproteinase inhibitors (MMPIs) (Abstract). Ann Oncol 9(suppl 2): 75, 1998
Neri A, Goggin B, Kolis S, Brekken J, Khelemskaya W, Gabriel L, Robinson SR, Webbers S, Wood AW, Appelt K, Shalinsky DR: Pharmacokinetics and efficacy of a novel matrix metalloproteinase inhibitor AG3340 in single agent and combination therapy against B16-F10 melanoma tumors developing in the lung after IV-tail vein implantation in C57BL/6 mice (Abstract). Proc Am Assoc Cancer Res 39: 302, 1998
Wojtowicz-Praga S, Torri J, Johnson M, Steen V, Marshall J, Ness E, Dickson R, Sale M, Rasmussen HS, Chiodo TA, Hawkins MJ: Phase I trial of marismastat, a novel matrix metalloproteinase inhibitor, administered orally to patients with advanced lung cancer. J Clin Oncol 16: 2150–2156, 1998
Hande K, Wilding G, Ripple G, Fry J, Arzoomanian R, Dixon M, Yuen G, Collier M: A phase I study of AG3340, a matrix metalloproease (MMP) inhibitor, in patients having advanced cancer (Abstract). Ann Oncol 9(suppl 2): 74, 1998
Krane SM: Clinical importance of metalloproteinase and their inhibitors. Ann NY Acad Sci 732: 1–10, 1994
Rosemurgy A Buckels J, Charnley R, Winston R, Steward W, Staddon A, Curtis L, Rugg T, Rasmussen H: A randomized study comparing marimastat to gemcitabine as first line therapy in patients with non-resectable pancreatic cancer (Abstract). Proc Am Soc Clin. Oncol 18: 261a, 1999
Fielding J, Scholefield R, Stuart R, Hawkins P, McCulloch P, Maughan T, Seymour M, Van Cutsem E, Thorlacius-Ussing O, Hovendal C: A randomized double-blind placebo-controlled study of marimastat in patients with inoperable gastric adenocarcinoma (Abstract). Proc Am Soc Clin Oncol 19: 240a, 2000
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by Bayer Inc.
Rights and permissions
About this article
Cite this article
Hirte, H., Stewart, D., Goel, R. et al. An NCIC-CTG phase I dose escalation pharmacokinetic study of the matrix metalloproteinase inhibitor BAY 12-9566 in combination with doxorubicin. Invest New Drugs 23, 437–443 (2005). https://doi.org/10.1007/s10637-005-2903-3
Issue Date:
DOI: https://doi.org/10.1007/s10637-005-2903-3