Summary
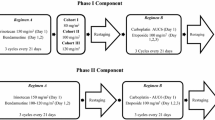
Rebeccamcyin analogue (RA) is an antitumor antibiotic that results in DNA intercalation and topoisomerase I and II inhibition. Phase I trials of the daily × 5 schedule and once every 3 week schedule have been completed. Antitumor activity was observed during the phase I trials. The purpose of this study is to primarily determine the response rate of 2 different schedules of administration of RA in patients with advanced non-small cell lung cancer (NSCLC) who had progressed on one prior chemotherapy regimen. Secondary endpoints were median and 1-year survival rates. A two-stage Simon design was employed for both arms of the study. Patients were randomly assigned to either of two RA treatment schedules of 500 mg/m2 as a 1 hr infusion repeated every 3 weeks (Arm A) or 140 mg/m2/day × 5 days repeated every 3 weeks (Arm B). Forty-two patients were randomized. No confirmed objective responses were seen. Stable disease was seen in 52% of patients on arm A and 37% on arm B. Median survival and 1 year survival rates were 5.6 months and 33.3% for arm A, 9.7 months and 42.1% for arm B respectively. Cox regression model demonstrated increased risk of death in patients younger than the age of 61 and for patients treated on arm A. RA failed to demonstrate a significant response rate in this disease setting, although the proportion of patients with stable disease and 1-year survival were encouraging and similar to other published studies of approved single agents for second-line therapy of NSCLC.
Similar content being viewed by others
References
Jemal A, Thomas A, Murray T, Thun M: Cancer statistics 2002. CA Cancer J Clin 52: 23–47, 2002
Vineis P, Alavanja M, Buffler P, Fontham E, Franceschi S, Gao YT, Gupta PC, Hackshaw A, Matos E, Samet J, Sitas F, Smith J, Stayner L, Straif K, Thun MJ, Wichmann HE, Wu AH, Zaridze D, Peto R, Doll R: Tobacco and cancer: Recent epidemiological evidence. J Natl Cancer Inst 96: 99–106, 2004
Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ: Cancer statistics 2004. CA Cancer J Clin 84-8-29, 2004
Idhe DC, Minna JD: Non-small cell lung cancer, part I: Biology, diagnosis, and staging. Curr Probl Cancer 15: 61–104, 1991
Juretic A, Sobat H, Samija M: Combined modality therapy of non-small lung cancers. Ann Oncol 10: 93–98. 1999
Boring C, Squires T, Tong T, Montgomery S: Cancer statistics 1994. CA Cancer J Clin 44: 7–26, 1994
Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R, Coughlin S, Kim Y, Berille J: Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18: 2095–2103, 2000
Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, Gandara D, Karp D, Vokes E, Kris M, Kim Y, Gamza F, Hammershaimb L: Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 18: 2354–2362, 2000
Kaneko T, Wong H, Okamoto KT, Clardy J: Two synthetic approaches to rebeccamycin. Tetrahedron Lett 26: 4015–4018, 1985
Nettledone DE, Doyle W, Krishnan B, Matsumoto K, Clardy J: Isolation and structure of rebeccamycin—a new antitumor antibiotic from Nocardia aerocolonigenes. Tetrahedron Lett 26: 4011–4014, 1985
Kaneko T, Wong H, Utzig J, Schurig J, Doyle T: Water-soluble derivatives of rebeccamycin. J Antibiot 40: 668–678, 1987
Merchant J, Tutsch K, Dresen A, Arzoomanian R, Alberti D, Feierabend C, Binger K, Marnoccha R, Thomas J, Cleary J, Wilding G: Phase I clinical and pharmacokinetic study of NSC 655649, a rebeccamycin analogue, given in both single-dose and multiple-dose formats. Clin Cancer Res 8: 2193–2201, 2002
Tolcher AW, Eckhardt SG, Kuhn J, Hammond L, Weiss G, Rizzo J, Aylesworth C, Hidalgo M, Patnaik A, Schwartz G, Felton S, Campbell E, Rowinsky EK: Phase I and pharmcokinetic study of NSC 655649, a rebeccamycin analogue with topoisomerase inhibitory properties. J Clin Oncol 19: 2937–2947, 2001
Dowlati A, Hoppel CL, Ingalls ST, Majka S, Li X, Sedransk N, Spiro T, Gerson SL, Ivy P, Remick SC: Phase I clinical and pharmacokinetic study of rebeccamycin analogue NSC 655649 given daily for five consecutive days. J Clin Oncol 19: 2309–2318, 2001
Simon R. Optimal two-stage designs for phase II clinical trials. Controlled Clin Trials 10: 1–10, 1989
National Cancer Institute: Common Toxicity Criteria, Version 2. Bethesda, MD, National Cancer Institute, 1999
Therase P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, Van Oosterom AT, Christian MC, Gwyther SG: New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3): 205–216, 2000
Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M, Muller T, Lim HL, Desch C, Szondy K, Gervais R, Shaharyar, Manegold C, Paul S, Paoletti P, Einhorn L, Bunn PA Jr: Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small cell lung cancer previously treated with chemotherapy. J Clin Oncol 22: 1589–1597, 2004
Shepherd FA, Pereira J, Ciuleanu TE, Tan EH, Hirsh V, Thongprasert S, Bezjak A, Tu D, Santabarbara P, Seymour L: A randomized placebo-controlled trial of erlotinib in patients with advanced non-small cell lung cancer (NSCLC) following failure of 1st line or 2nd line chemotherapy. A National Cancer Institute of Canada Clinical Trials Group Trial. J Clin Oncol, 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition). Vol 22, No. (14S) (July 15 Suppl.) p. 7022, 2004
Burstein HJ, Overmoyer B, Gelman R, et al.: Rebeccamycin analog for refractory breast cancer: A randomized phase II trial. J Clin Oncol, 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition). Vol. 22, No. (14S) (July 15 Suppl.), p. 547, 2004
Dowlati A, Posey J, Ramanathan RK, Rath, L, Sellers A, Schmotzer A, Ingalls S, Hoppel C, Ivy P, Remick SC: Multicenter phase II and pharmacokinetic study of rebeccamycin analogue (RA) in advanced biliary cancers. Proc Am Soc Clin Oncol 22: abstract 1070, 2003
Lilenbaum R: Management of advanced non-small-cell lung cancer in elderly populations. Clin Lung Cancer 5: 169–173, 2003
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dowlati, A., Chapman, R., Subbiah, S. et al. Randomized phase II trial of different schedules of administration of rebeccamycin analogue as second line therapy in non-small cell lung cancer. Invest New Drugs 23, 563–567 (2005). https://doi.org/10.1007/s10637-005-0754-6
Issue Date:
DOI: https://doi.org/10.1007/s10637-005-0754-6