Abstract
Purpose
To compare the characteristics of the photopic negative response (PhNR) between the focal macular and full-field electroretinograms (ERGs) in monkeys.
Methods
Both focal macular and full-field photopic ERGs were recorded in four cynomolgus monkeys under identical stimulus and recording conditions except for which area of the retina was illuminated. The luminance and duration of red flash stimuli were varied in the presence of steady blue background illumination. These ERGs were recorded before and after intravitreal injection of tetrodotoxin (TTX).
Results
Several differences were identified between the focal macular and full-field ERGs, including: (1) The PhNR/b-wave amplitude ratio was higher in the focal macular than in the full-field ERGs, and (2) the stimulus threshold of the focal macular PhNR was lower than that of the full-field PhNR. For both macular and full-field stimulation conditions, (1) PhNR amplitude generally increased with increasing stimulus luminance; (2) PhNR implicit time was independent of the stimulus luminance; (3) PhNR amplitude and implicit time increased with increasing stimulus duration up to 50 ms, while a further increase in stimulus duration produced no change in amplitude or implicit time; and (4) PhNR amplitude was selectively attenuated by TTX.
Conclusions
Both the focal macular and full-field PhNRs reflect the functional properties of the inner retina including the retinal ganglion cells (RGCs). Relative to the b-wave, the contribution is weighted more heavily in the focal macular than in the full-field PhNR. Furthermore, these results support the idea that the focal macular PhNR can be an indicator of the function of the macular RGCs.
Similar content being viewed by others
Introduction
The photopic negative response (PhNR) is a corneal negative potential that follows the b-wave in the photopic electroretinogram (ERG). The PhNR obtained in response to full-field stimulation (i.e., the full-field PhNR) has been shown to originate mainly from the neural activity of the retinal ganglion cells (RGCs) in monkeys and humans [1, 2]. Several research studies have shown selectively or predominantly diminished full-field PhNRs in patients with disease of the optic nerve or inner retina affecting the RGCs, such as optic nerve atrophy [3–6] or glaucoma [2, 7]. The PhNR, therefore, provides an objective functional test for these conditions.
Focal ERGs elicited under photopic condition also include a PhNR component (i.e., the focal PhNR). Patients with optic nerve atrophy who manifested central scotoma may exhibit normal full-field PhNR but diminished focal macular PhNR amplitudes, indicating the potential for the focal macular PhNR to detect localized optic nerve atrophy [8]. Furthermore, when Machida and colleagues recorded focal and full-field PhNRs from patients with glaucoma, they showed that the detection sensitivity was significantly higher for the focal PhNR than for the full-field PhNR in those patients with early-stage glaucoma [9]. These clinical findings suggest that the focal macular PhNR could be particularly useful for detecting localized RGC dysfunction, especially related to loss of central vision, and for stratifying the risk of disease progression.
In monkeys, Kondo and colleagues established a system for recording the focal PhNR elicited by red stimuli presented against a steady blue background and used pharmacological treatment to demonstrate that the focal macular PhNR originated from the action potentials of inner retinal neurons [10]. They also compared the response characteristics of the focal macular and full-field PhNRs obtained from monkeys. However, a study comparing the response properties of the focal macular and full-field PhNRs obtained under the comparable stimulus and recording conditions from the same subjects has not been previously published. Here we report the results of this analysis, in which we thoroughly compare the response characteristics of the focal macular and full-field PhNRs. To accomplish this, we obtained the focal macular and full-field PhNRs recorded under identical stimulus and recording conditions from monkeys, a species that has human-like macula lutea. Focal macular and full-field PhNRs were also recorded after an intravitreal injection of tetrodotoxin (TTX) to compare the relative contributions of the inner retina to each. Our results suggest that the macular PhNR is advantageous for detection of RGC dysfunction in the macula.
Methods
Animals
A total of four cynomolgus monkeys (Macaca fascicularis) at 6 years of age were used in this study. The animals were housed individually in stainless steel cages (W 59 cm × D 87 cm × H 102 cm) in an animal study room where the environmental conditions were set as follows: room temperature, 24 °C; relative humidity, 60 %; and illumination, 12-h lighting (7:00–19:00) at 300 lx. The animals were fed 100 g/animal/day of pellet food for monkeys (PS-A, Oriental Yeast Co., Ltd., Tokyo, Japan). Chlorinated municipal tap water was supplied ad libitum from a feed-water nozzle. Environmental enrichment was provided, including toys and puzzled feeding apparatus.
Study design
Full-field and focal macular photopic ERGs were serially recorded from the left eye of each of the four animals, as described below. After an interval of 1 week or more, these ERGs were also recorded in the same manner after an intravitreous injection of TTX.
Animal preparation for ERG recording
The animals were anesthetized with intramuscular injection of ketamine HCl (Ketalar Intramuscular 500 mg, Daiichi Sankyo Co., Ltd., Tokyo, Japan) (10 mg/kg initial dose, followed by maintenance doses of 10–20 mg/kg/h) and 0.6 mg/kg xylazine HCl (Celactal, Bayer Medical Ltd., Osaka, Japan). The pupils were dilated with topical 0.5 % tropicamide and 0.5 % phenylephrine HCl (Mydrin-P ophthalmic solution, Santen Pharmaceutical Co., Ltd., Osaka, Japan); the corneas were anesthetized with topical 0.4 % oxybuprocaine HCl (Benoxil ophthalmic solution 0.4 %, Santen Pharmaceutical Co., Ltd.) and protected with topical hydroxyethylcellulose (Scopisol solution for eye, Takeda Chemical Industries, Ltd., Osaka, Japan).
Visual stimulation
Full-field stimulation was produced with a Ganzfeld stimulator (BigShot Ganzfeld, LKC Technologies, Inc., Gaithersburg, MD, USA). The red stimulus and blue background illuminations were generated by the red (λ max = 627 nm) and blue (λ max = 470 nm) light-emitting diodes (LEDs), respectively. This color combination was selected based on a prior report demonstrating that the red stimuli and blue background illumination are effective in eliciting large PhNR, especially at weak to moderate stimulus levels [11].
The system to elicit the focal macular ERGs was basically identical to that established by Kondo et al. and consisted of a modified infrared fundus camera and a stimulator that controlled the LEDs used for the stimulus and background illumination (ER-80, Kowa Co., Ltd., Aichi, Japan) [10]. The red stimulus spot could be varied from 5° to 15° in diameter, while the blue background field covered 45° of the central retina. The light sources for stimulus and background illumination of the focal ERG system were replaced with a red LED (λ max = 627 nm; LXK2-PD12-R00, Philips Lumileds Lighting Company, San Jose, CA, USA) and a blue LED (λ max = 470 nm; NS6B083, Nichia Corporation, Tokushima, Japan), respectively, in order to achieve identical stimulus conditions to that for the full-field ERGs except for which area of the retina was illuminated.
The luminance levels of the stimulus and background illumination generated in these two systems were measured by a calibrated photometer (IL1700, International Light Technologies, Inc., Peabody, MA, USA) and optical detectors (SED033/Y, SED033/Y/R and SED033/ZCIE/R, International Light Technologies, Inc.). For the macular ERG system, the luminance levels were measured at the position of the corneal surface and then converted to the values at the retinal surface.
ERG recording and analysis
All ERGs were recorded from the corneal surface of the left eye of each animal with a Burian-Allen bipolar contact lens electrode (Hansen Ophthalmic Development Laboratories, Coralville, IA, USA). A ground electrode (TN208-016, Unique Medical Co., Ltd., Tokyo, Japan) was attached to the parietal region of the scalp. The background for both full-field and focal macular ERGs was fixed at 110 scot cd/m2 (7 phot cd/m2), which is sufficient to suppress the response of rod photoreceptors to superimposed stimuli. Stimulus luminance and duration varied from 1 to 56 phot cd/m2 and from 5 to 150 ms, respectively. In a preliminary study using a different cynomolgus monkey, the 15° central area of the macula of one eye was damaged by focal laser photocoagulation. When this eye was centrally stimulated with red 15° stimulus spots of 150-ms duration superimposed of the blue background, no reproducible waveform was obtained up to stimulus luminance levels of 82 phot cd/m2. Above this level, responses were noted and attributed to stray light responses from distant retinal regions. Therefore, the highest stimulus luminance used here (56 phot cd/m2) was sufficiently below the point where a stray light response might be obtained. The stimulus strength was also expressed in an energy unit (i.e., phot cd s/m2, for brief flashes of 30 ms or shorter).
After completing the initial setup procedures, the eye was adapted to the blue background light for 10 min. In a given trial, full-field ERGs were elicited by red light stimuli presented at 2 Hz and superimposed upon the steady blue background field. A total of 20 responses were averaged for each recording. When the full-field protocol was completed, we obtained focal macular ERGs to 15° red stimuli presented at 2 Hz and superimposed upon the blue background field. Because focal macular ERGs had smaller amplitude, we averaged 100–200 successive focal macular responses. Responses of the full-field ERGs and the focal macular ERGs were amplified 10,000 and 20,000 times, respectively, using an amplifier band pass of 1–1000 Hz. The amplified signals were stored in the evoked potential test equipment (MEB-9104, Nihon Kohden Corporation, Tokyo, Japan). Recording sessions were completed within 3 h (average duration of 1.8 h). During the recording session, rectal temperature of the animal was maintained to a minimum of 37.0 °C using an electric heating system (ATC-101B, Unique Medical Co., Ltd.).
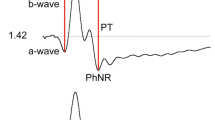
The amplitude of the PhNR was measured from the baseline to the bottom of the negative trough following the b-wave for brief (≤30 ms) flashes or from the b-wave peak to the bottom of the negative trough following the b-wave for the long-duration (≥50 ms) flashes [1, 10, 11]. The amplitude of the b-wave was measured from the first negative trough to the next positive peak. The PhNR/b-wave amplitude ratio [12] was calculated as the ratio of the PhNR amplitude to the b-wave amplitude. In addition, implicit time of the PhNR was measured from the stimulus onset to the bottom of the negative trough following the b-wave for the long-duration flashes of 150 ms, in which the PhNR after the onset of the long-duration stimuli (i.e., the PhNRon response [1]) was well isolated in both the focal macular and full-field ERGs. The PhNR implicit time was also measured for the strong flashes of 56 phot cd/m2. For brief and low luminance stimuli, the exact PhNR trough of the full-field ERGs was difficult to gauge due to intrusion by a small positive component, presumably the i-wave. No measurement of the waveform components was taken in the ERGs recorded after intravitreal injection of TTX.
Drug administration
Intravitreal injection of TTX was conducted in four animals. TTX citrate (Wako Pure Chemical Industries, Ltd., Osaka, Japan) was dissolved in distilled water at a concentration of 160 μM, followed by filter sterilization. Immediately after preparation of the TTX, the animal was anesthetized with intramuscular injection of 10 mg/kg ketamine HCl (Ketalar Intramuscular 500 mg, Daiichi Sankyo Co., Ltd.), and the surface of the left eye was anesthetized with topical 0.4 % oxybuprocaine HCl (Benoxil ophthalmic solution 0.4 %, Santen Pharmaceutical Co., Ltd.). A disposable syringe attached to a 30-gauge needle (Myshot, Nipro Corp., Osaka, Japan) was used to inject 50 μL of the TTX solution into the vitreous of the left eye. The injection was made through the pars plana approximately 4 mm posterior to the limbus. The concentration of TTX in the vitreous would be approximately 4 μM, assuming that the vitreous volume is 2 mL in monkeys. ERG recording after intravitreal injection was initiated approximately 2 h after the injection.
Statistics
For statistical analyses of the luminance–response and duration–response functions of the PhNR/b-wave amplitude ratio and the PhNR implicit time, a two-way ANOVA was used to assess the differences between the focal macular and full-field ERGs. The differences were considered to be statistically significant when P was <0.05. No post hoc comparison followed the two-way ANOVA, since statistical significance was not observed in the interaction between luminance and response or between duration and response.
Results
Effect of stimulus luminance
Typical waveforms of the focal macular and full-field ERGs elicited with flash stimuli at different luminance levels and durations are shown in Fig. 1. The PhNRs appeared to be enhanced with increasing stimulus luminance for all stimulus durations both in the focal macular and in full-field ERGs. For stimulus durations of 5, 10 and 30 ms, PhNR amplitudes matched across the luminance range examined (Fig. 2a, left). On the other hand, for the full-field PhNRs the intensity–amplitude curve for 30-ms stimuli shifted toward approximately twofold stronger intensity compared to those for shorter stimuli of 5 and 10 ms (Fig. 2a, right). For both brief and long-duration stimuli, the stimulus threshold of the focal macular PhNR was lower than that of the full-field PhNR.
Typical waveforms of the focal macular ERGs and the full-field ERGs in a monkey elicited with red flash stimuli at different luminance levels and durations in the presence of steady blue background illumination. The dotted vertical lines and the rectangles at the bottom of the traces indicate the onset and duration of stimuli, respectively. Each trace represents the average of 100 to 200 responses for the focal macular ERGs and 20 responses for the full-field ERGs. The PhNR was enhanced with increasing stimulus luminances at all stimulus durations in both the focal macular and full-field ERGs
Comparison of intensity–response functions of the PhNR amplitude between the focal macular and full-field ERGs in monkeys. The focal macular and full-field ERGs were elicited with red flash stimuli at different luminance levels and durations in the presence of steady blue background illumination. The PhNR amplitudes in the focal macular ERG (left panel) and those in the full-field ERG (right panel) are plotted as a function of stimulus intensity for brief flashes (a) and stimulus luminance for long flashes (b). All data are expressed as mean ± SD of four animals. Note that the intensity–response curve for 30-ms stimuli in the full-field PhNRs shifted toward stronger intensity
Figure 3 compares luminance–response functions for the PhNR and b-wave components obtained for focal macular (left panel) and full-field (middle panel) stimuli. For all flash luminance levels, PhNR amplitude was larger than b-wave amplitude for focal macular ERGs. For full-field stimuli, PhNR amplitudes were comparable to or smaller than the b-wave amplitudes. In comparison with the full-field ERGs, the PhNR/b-wave amplitude ratio was significantly higher for focal macular ERGs, for both brief and long-duration flashes (Fig. 3, right panel).
Comparison of the intensity–response functions of the b-wave and PhNR amplitudes between the focal macular and full-field ERGs in monkeys. The focal macular and full-field ERGs were elicited with red flash stimuli at different luminance levels and durations in the presence of steady blue background illumination. The amplitudes of the b-wave and PhNR in the focal macular ERG (left panel) and those in the full-field ERG (middle panel) are plotted as a function of stimulus intensity for brief flashes (a) and stimulus luminance for long flashes (b). The PhNR/b-wave amplitude ratios were calculated as the ratio of the PhNR amplitude to the b-wave amplitude and are plotted in the same manner (right panel). All data are expressed as mean ± SD of four animals. The PhNR amplitude was larger than the b-wave amplitude in the focal macular ERGs for both brief and long flashes; in contrast, the PhNR amplitude was comparable to or smaller than the b-wave amplitude in the full-field ERGs. The values of the PhNR/b-wave amplitude ratio in the focal macular ERGs were significantly higher than those in the full-field ERGs (two-way ANOVA, *P < 0.01) for both brief and long flashes. Statistically significant interaction was not observed between response and stimulus intensity or between response and stimulus luminance
The implicit times of the PhNR for long-duration flashes were significantly longer in the focal macular ERG than in the full-field ERG (Fig. 4). Across stimulus luminance, the mean implicit time of the focal macular PhNR was approximately 25 ms longer than that of the full-field PhNR.
Comparison of intensity–response functions of the PhNR implicit times between the focal macular and full-field ERGs in monkeys. The focal macular and full-field ERGs were elicited with red flash stimuli at different stimulus luminances in the presence of steady blue background illumination. The implicit times of the PhNR are plotted as a function of stimulus luminance. Data are expressed as mean ± SD of four animals. The implicit times of the focal macular PhNR were significantly longer in comparison with the values in the full-field PhNR by the two-way ANOVA (*P < 0.01). The mean implicit times of the PhNR were approximately 25 ms longer in the focal macular ERG than in the full-field ERG across the stimulus luminance levels utilized; no statistically significant interaction was detected between PhNR implicit time and stimulus luminance
Effect of stimulus duration
Typical waveforms of the focal macular and full-field ERGs elicited with flash stimuli of different durations are shown in Fig. 5. In addition to the PhNRs following the b-wave after the stimulus onset, we noted additional negative-going slow waves after the offset of the long-duration stimuli, the PhNRoff response [1], in both the focal macular and full-field ERGs (Fig. 5, asterisks). These were not analyzed further.
Typical waveforms of the focal macular and full-field ERGs in a monkey elicited with red flash stimuli of different durations at a fixed luminance of 56 phot cd/m2 in the presence of steady blue background illumination. The dotted vertical lines and the rectangles at the bottom of the traces indicate the onset and duration of stimuli, respectively. The dashed vertical lines signify the time points of the PhNR trough. Each trace represents the average of 100 to 200 responses for the focal macular ERGs and 20 responses for the full-field ERGs. Note that the negative slow waves after the offset of the long-duration stimuli were identified both in the focal macular and in full-field ERGs (asterisks)
These waveforms were measured to define duration–response functions of the PhNR (Fig. 6). For both focal macular and full-field conditions, PhNR amplitude increased with stimulus duration up to 50 ms after which it maintained a stable level (Fig. 6a). A similar dependence was noted for implicit time, which increased up to the asymptote achieved for 50-ms duration stimuli (Fig. 6b). PhNR implicit times were significantly longer for focal macular as compared to full-field PhNRs; the implicit times of the focal macular PhNR were approximately 25 ms longer than those of the full-field PhNR across the stimulus duration range examined. The ratio of PhNR to b-wave amplitude was significantly higher in the focal macular ERGs than in the full-field ERGs for all stimulus durations (Fig. 6c).
Comparison of duration–response functions of the amplitude and implicit time of the PhNR and the PhNR/b-wave amplitude ratio between the focal macular and full-field ERGs in monkeys. The focal macular and full-field ERGs were elicited with red flash stimuli of different durations at a fixed luminance of 56 phot cd/m2 in the presence of steady blue background illumination. The (a) amplitudes and (b) implicit times of the PhNR and (c) the PhNR/b-wave amplitude ratios are plotted as a function of stimulus duration. Data are expressed as mean ± SD of four animals. The amplitude and implicit time of the PhNR generally increased with increasing stimulus duration up to 50 ms both in the focal macular and in full-field ERGs, but further increase in stimulus duration produced no further increase in the PhNR amplitude or implicit time. The values of the PhNR implicit time and PhNR/b-wave amplitude ratio were significantly higher in the focal macular ERG compared with the full-field ERG by the two-way ANOVA (*P < 0.01). Statistically significant interaction was not detected between response and stimulus duration
Effect of intravitreal TTX
Figure 7 compares a duration series of ERGs obtained before (gray tracings) and after (black tracings) intravitreal administration of TTX. For all stimulus conditions, the early ERG waveform was largely unaffected. The b-waves of the focal macular ERG were enhanced by TTX, indicating that the onset of the negative polarity PhNR acts to truncate this positive ERG component. A similar effect was not observed for full-field ERGs, where the b-waves were generally comparable for TTX-treated and untreated eyes. In comparison, the PhNR was selectively reduced for all stimulation conditions (Fig. 7, arrow heads). TTX also reduced the amplitude of the additional slower negative-going waves (Fig. 7, asterisks), indicating that these also reflect the activity of spiking neurons.
Typical waveforms of the focal macular and full-field ERGs in a monkey elicited with red flash stimuli at a fixed luminance of 56 phot cd/m2 in the presence of steady blue background illumination before and after intravitreal injection of TTX. The dotted vertical lines and the rectangles at the bottom of the traces indicate the onset and duration of stimuli, respectively. The responses before injection (gray traces) are superimposed on those obtained after injection (black traces). Each trace represents the average of 100 to 200 responses for the focal macular ERGs and 20 responses for the full-field ERGs. TTX markedly attenuated the PhNRs that follow the b-wave (arrow heads), and other negative-going slow waves identified after the offset of the long-duration stimuli (asterisks) in both the focal macular and full-field ERGs
Discussion
Several lines of evidence from clinical studies have implied that the focal macular PhNR can be utilized to detect localized and early loss of the macular RGC function in patients with inner retinal diseases [8, 13, 14]. However, knowledge about the response characteristics of the focal macular PhNR still needs to develop compared to that of the full-field PhNR. In the present study, we examined the monkey ERG and noted a number of similarities between focal macular and full-field PhNRs. First, PhNR amplitude generally increased with increasing stimulus luminance. Second, PhNR implicit time did not change across stimulus luminance. Third, both the amplitude and implicit time of the PhNR increased with increasing stimulus duration, up to a maximum after which further increases in flash duration had no effect. Fourth, the PhNR was identified after both the onset and the offset of the stimulus for long-duration stimuli. Lastly, the PhNR was markedly attenuated by intravitreal TTX, which selectively blocks Na+-dependent action potentials, which mainly occur in the RGCs [15, 16]. These similarities in response characteristics of the PhNR between the focal macular and full-field ERGs in monkeys suggest that the focal macular PhNR is a local analogue of the full-field PhNR and therefore can be an indicator of the function of the inner retina in the macula.
We also observe differences in the PhNR between the focal macular and full-field ERGs. In comparison with other ERG components, the PhNR was relatively larger in the focal macular ERGs than in full-field ERGs. As a result, the PhNR/b-wave amplitude ratio was higher in the focal macular ERGs than in the full-field ERGs. This finding is consistent with the prior work by Kondo and colleagues [10] who reported that focal PhNRs recorded from monkey macula appeared to be relatively larger when compared with the full-field PhNRs obtained in earlier monkey studies. We also found that the difference in appearance of the PhNR between the focal macular and full-field ERGs (i.e., the deeper and wider PhNR in the focal macular ERGs especially for long-duration flashes, see Figs. 5, 7) became smaller after TTX injection, indicating that this difference in the PhNR appearance was generally due to the difference in contributions from the TTX-sensitive components between the focal macular and full-field ERGs. Furthermore, the stimulus threshold of the PhNR in the focal macular ERGs was lower than that in the full-field ERGs. Although the exact mechanisms underlying the relatively larger amplitude and lower stimulus threshold of the macular PhNR remain to be elucidated, they could be attributable to the higher density of the RGCs compared with the cone photoreceptors in the central retina in monkeys [17–19]. The observed differences between the focal macular and full-field PhNRs indicate that functional contribution of the inner retina including the RGCs is greater in the focal macular ERG than in the full-field ERG in monkeys. Therefore, our results support the idea that the PhNR elicited from the macula is advantageous for detection of macular RGC dysfunction, consistent with recent studies [14, 20].
We observed other differences in the response properties of the PhNR between the focal macular and full-field ERGs. The amplitude of the focal macular PhNR was dependent on the stimulus intensity for brief flashes up to 30 ms, indicated by the closely matched intensity–response functions obtained to 5-, 10- and 30-ms stimuli (Fig. 2). On the other hand, the intensity–amplitude functions of the full-field PhNRs obtained to 30-ms stimuli were shifted to the right from those obtained to 5- or 10-ms stimuli. This finding indicates that more intense light is required to obtain an equivalent response amplitude for a 30 ms as compared to a 5- or 10-ms duration stimulus in the full-field PhNRs. The reasons for this difference are yet to be determined. However, this might be explained by a topographic variation of the integration time of the cone photoreceptors across the retina, with central cones having a longer integration time than peripheral cones. Of course, this observation could be secondary to a difference in the time of b-wave appearance, specifically a more prolonged b-wave in the full-field ERG for 30-ms stimuli as compared with that in the macular ERG. In addition, the b-waves of the focal macular ERG were enhanced by TTX, while a similar effect was not observed for full-field ERGs. This finding suggests that the contributions from spiking neurons in the inner retina compared with those from the ON-bipolar cells, which are the main generators of the b-wave in monkeys [21], are relatively earlier in the focal macular ERG than in the full-field ERG.
In conclusion, the clear differences between the focal macular and full-field PhNRs recorded in monkeys in this study indicate that functional contribution of the inner retina, compared to the contribution of the retinal layer generating the b-wave, is greater in the focal macular ERG than in the full-field ERG. This observation, and the documented similarities between these responses, supports the idea that the focal macular PhNR is a local analogue of the full-field PhNR and can therefore be used to monitor inner retinal function in the macula.
References
Viswanathan S, Frishman LJ, Robson JG, Harwerth RS, Smith EL III (1999) The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci 40(6):1124–1136
Viswanathan S, Frishman LJ, Robson JG, Walters JW (2001) The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci 42(2):514–522
Gotoh Y, Machida S, Tazawa Y (2004) Selective loss of the photopic negative response in patients with optic nerve atrophy. Arch Ophthalmol 122(3):341–346. doi:10.1001/archopht.122.3.341
Rangaswamy NV, Frishman LJ, Dorotheo EU, Schiffman JS, Bahrani HM, Tang RA (2004) Photopic ERGs in patients with optic neuropathies: comparison with primate ERGs after pharmacologic blockade of inner retina. Invest Ophthalmol Vis Sci 45(10):3827–3837. doi:10.1167/iovs.04-0458
Moon CH, Hwang SC, Kim BT, Ohn YH, Park TK (2011) Visual prognostic value of optical coherence tomography and photopic negative response in chiasmal compression. Invest Ophthalmol Vis Sci 52(11):8527–8533. doi:10.1167/iovs.11-8034
Wang J, Cheng H, Hu YS, Tang RA, Frishman LJ (2012) The photopic negative response of the flash electroretinogram in multiple sclerosis. Invest Ophthalmol Vis Sci 53(3):1315–1323. doi:10.1167/iovs.11-8461
Machida S, Gotoh Y, Toba Y, Ohtaki A, Kaneko M, Kurosaka D (2008) Correlation between photopic negative response and retinal nerve fiber layer thickness and optic disc topography in glaucomatous eyes. Invest Ophthalmol Vis Sci 49(5):2201–2207. doi:10.1167/iovs.07-0887
Tamada K, Machida S, Yokoyama D, Kurosaka D (2009) Photopic negative response of full-field and focal macular electroretinograms in patients with optic nerve atrophy. Jpn J Ophthalmol 53(6):608–614. doi:10.1007/s10384-009-0731-2
Machida S, Tamada K, Oikawa T, Gotoh Y, Nishimura T, Kaneko M, Kurosaka D (2011) Comparison of photopic negative response of full-field and focal electroretinograms in detecting glaucomatous eyes. J Ophthalmol. doi:10.1155/2011/564131
Kondo M, Kurimoto Y, Sakai T, Koyasu T, Miyata K, Ueno S, Terasaki H (2008) Recording focal macular photopic negative response (PhNR) from monkeys. Invest Ophthalmol Vis Sci 49(8):3544–3550. doi:10.1167/iovs.08-1798
Rangaswamy NV, Shirato S, Kaneko M, Digby BI, Robson JG, Frishman LJ (2007) Effects of spectral characteristics of Ganzfeld stimuli on the photopic negative response (PhNR) of the ERG. Invest Ophthalmol Vis Sci 48(10):4818–4828. doi:10.1167/iovs.07-0218
Fortune B, Bui BV, Cull G, Wang L, Cioffi GA (2004) Inter-ocular and inter-session reliability of the electroretinogram photopic negative response (PhNR) in non-human primates. Exp Eye Res 78(1):83–93
Nakamura H, Hangai M, Mori S, Hirose F, Yoshimura N (2011) Hemispherical focal macular photopic negative response and macular inner retinal thickness in open-angle glaucoma. Am J Ophthalmol 151(3):494–506. doi:10.1016/j.ajo.2010.09.018
Machida S, Kaneko M, Kurosaka D (2015) Regional variations in correlation between photopic negative response of focal electroretinograms and ganglion cell complex in glaucoma. Curr Eye Res 40(4):439–449. doi:10.3109/02713683.2014.922196
Hille B (1966) Common mode of action of three agents that decrease the transient change in sodium permeability in nerves. Nature 210(5042):1220–1222
Bloomfield SA (1996) Effect of spike blockade on the receptive-field size of amacrine and ganglion cells in the rabbit retina. J Neurophysiol 75(5):1878–1893
Stone J, Johnston E (1981) The topography of primate retina: a study of the human, bushbaby, and new- and old-world monkeys. J Comp Neurol 196(2):205–223. doi:10.1002/cne.901960204
Perry VH, Cowey A (1985) The ganglion cell and cone distributions in the monkey’s retina: implications for central magnification factors. Vis Res 25(12):1795–1810
Curcio CA, Sloan KR Jr, Packer O, Hendrickson AE, Kalina RE (1987) Distribution of cones in human and monkey retina: individual variability and radial asymmetry. Science 236(4801):579–582
Kaneko M, Machida S, Hoshi Y, Kurosaka D (2015) Alterations of photopic negative response of multifocal electroretinogram in patients with glaucoma. Curr Eye Res 40(1):77–86. doi:10.3109/02713683.2014.915575
Sieving PA, Murayama K, Naarendorp F (1994) Push-pull model of the primate photopic electroretinogram: a role for hyperpolarizing neurons in shaping the b-wave. Vis Neurosci 11(3):519–532
Acknowledgments
The authors thank Hidetaka Kudo of Mayo Corporation for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Human and animal rights
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the Institutional Animal Care and Use Committee of Daiichi Sankyo Co. Ltd. This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kinoshita, J., Takada, S., Iwata, N. et al. Comparison of photopic negative response (PhNR) between focal macular and full-field electroretinograms in monkeys. Doc Ophthalmol 132, 177–187 (2016). https://doi.org/10.1007/s10633-016-9538-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-016-9538-x