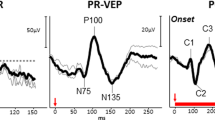
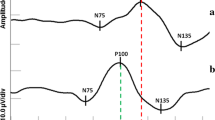
Abstract
Background and Methods In routine clinical evaluation of optic neuritis and chiasmal tumours, pattern electroretinography and visual evoked potentials (VEPs) to pattern-reversal stimulation are useful examinations. Similarly, in achiasmia and ocular albinism, VEPs to flash and pattern-onset stimulation provide relevant information. Results The role of visual electrophysiology in these diseases is to assess potential dysfunction of the visual pathway: (a) at the acute stage of optic neuritis, to determine the magnitude of conduction block of the optic nerve fibres; (b) at the clinical recovery stage of optic neuritis, to determine optic nerve conduction delay due to demyelination, and to follow possible remyelination; (c) at the recovery of optic neuritis when visual acuity does not normalise, to define loss of optic nerve fibres and retrograde degeneration of retinal ganglion cells; (d) in tumours at the chiasm, to detect abnormal conduction along the crossed and/or uncrossed fibres; and (e) in achiasmia or albinism, which are both congenital disorders associated with nystagmus, to detect achiasmia and absence of or reduced optic nerve fibre decussation at the chiasm, or to detect ocular albinism and excess of optic nerve fibre decussation at the chiasm. In optic neuritis, two recent examinations have been used to detect retrograde axonal degeneration: photopic negative response of the electroretinogram, to assess dysfunction of ganglion cell axons; and optic coherence tomography, to measure thinning of the retinal nerve fibre layer. In optic neuritis, multifocal VEPs provide a promising clinical examination, because this can show areas that are associated with normal or abnormal optic nerve fibre function. Conclusions Visual electrophysiology defines function of the visual pathway and is relevant: (1) in optic neuritis, when visual acuity does not recover well; (2) in tumours of the chiasm with normal visual fields, as in paediatric patients who cannot adequately perform perimetry; and (3) in children with congenital nystagmus and suspected achiasmia or ocular albinism.







Similar content being viewed by others
References
Halliday AM, Mc Donald WI, Mushin J (1972) Delayed visual evoked response in optic neuritis. Lancet 1:982–985
Halliday AM, McDonald WI, Mushin J (1973) Visual evoked response in diagnosis of multiple sclerosis. Br Med J 4:661–664
Halliday AM, Halliday E, Kriss A, McDonald WI, Mushin J (1976) The pattern-evoked potential in compression of the anterior visual pathways. Brain 99:357–374
Odom JV, Bach M, Brigell M, Holder GE, McCulloch DL, Tormene AP, Vaegan (2010) ISCEV standard for clinical visual evoked potentials (2009 update). Doc Ophthalmol 120:111–119
Bach M, Brigell MG, Hawlina M, Holder GE, Johnson MA, McCulloch DL, Meigen T, Viswanathan S (2013) ISCEV standard for clinical pattern electroretinography (PERG)—2012 update. Doc Ophthalmol 126:1–7
Holder GE, Celesia GG, Miyake Y, Tobimatsu S, Weleber RG (2010) International Federation of Clinical Neurophysiology: recommendations for visual system testing. Clin Neurophysiol 121:1393–1409
Holder GE, Gale RP, Acheson JF, Robson AG (2009) Electrodiagnostic assessment in optic nerve disease. Curr Opin Neurol 22:3–10
Holder GE (2001) The pattern electroretinography (PERG) and an integrated approach to visual pathway diagnosis. Prog Retin Eye Res 20(4):531–561
Viswanathan S, Frishman LJ, Robson JG, Walters JW (2001) The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci 42:514–522
Sustar M, Cvenkel B, Brecelj J (2009) The effect of broadband and monochromatic stimuli on the photopic negative response of the electroretinogram in normal subjects and in open-angle glaucoma patients. Doc Ophthalmol 118:167–177
Brecelj J, Cunningham K (1985) Occipital distribution of foveal half-field responses. Doc Ophthalmol 59:157–165
Brecelj J, Kakigi R, Koyama S, Hoshiyama M (1998) Visual evoked magnetic responses to central and peripheral stimulation: simultaneous VEP recordings. Brain Topogr 10:227–237
Brecelj J (1991) Visual evoked potentials and the localization of visual pathway lesions. Spektrum Augenheilkd 5:114–122
Fortune B, Hood DC (2003) Conventional pattern-reversal VEPs are not equivalent to summed multifocal VEPs. Invest Ophthalmol Vis Sci 44:1364–1375
Plant GT (2008) Optic neuritis and multiple sclerosis. Curr Opin Neurol 21:16–21
Shams PN, Plant GT (2009) Optic neuritis: a review. Int MS J 16:82–89
Kidd D, Burton B, Plant GT, Graham EM (2003) Chronic relapsing inflammatory optic neuropathy (CRION). Brain 126:276–284
Kriss A, Francis DA, Cuendet F, Halliday AM, Taylor DSI, Wilson J, Keast-Butler J, Batchelor JR, McDonald WI (1988) Recovery after optic neuritis in childhood. J Neurol Neurosurg Psychiatry 51:1253–1258
Wilejto M, Shroff M, Buncic JR, Kennedy J, Goia C, Banwell B (2006) The clinical features, MRI findings, and outcome of optic neuritis in children. Neurology 67:258–262
Jones SJ, Brusa A (2003) Neurophysiological evidence for long-term repair of MS lesions: implications for axon protection. J Neurol Sci 206:193–198
Brecelj J, Denišlič M, Prevec TS, Štrucl M (1981) Visual evoked potentials in the assessment of subclinical demyelinating lesions of the visual pathway (in Slovene). Zdrav Vestn 50:145–149
Brecelj J, Kriss A (1989) Pattern reversal VEPs in optic neuritis. Advantages of central and peripheral half-field stimulation. Neuro-Ophthalmology 9:55–63
Brecelj J, Štrucl M, Hawlina M (1990) Central fiber contribution to W-shaped visual evoked potentials in patients with optic neuritis. Doc Ophthalmol 75:155–163
Brecelj J, Stirn-Kranjc B Tekavčič-Pompe M (1998) A VEP and PERG study in children with suspected optic neuritis. In: Hashimoto I, Kakigi R (eds) Recent advances in human neurophysiology: Proceedings of the 6th international evoked potentials symposium, Okazaki, Japan 21–25 March 1998. Elsevier, Amsterdam, pp 496–501
Tekavčič-Pompe M, Stirn-Kranjc B, Brecelj J (2003) Optic neuritis in children—clinical and electrophysiological follow-up. Doc Ophthalmol 107:261–270
Liščić RM, Brecelj J (2004) Visual evoked potentials in multiple sclerosis patients treated with interferon beta-1a. Croat Med J 45(3):323–327
Holder GE (1991) The incidence of abnormal pattern electroretinography in optic nerve demyelination. Electroenceph Clin Neurophysiol 78:18–26
Rinalduzzi S, Brusa A, Jones SJ (2001) Variation of visual evoked potential delay to stimulation of central, nasal, and temporal regions of the macula in optic neuritis. J Neurol Neurosurg Psychiatry 70:28–35
Brusa A, Jones SJ, Plant GT (2001) Long-term remyelination after optic neuritis. A 2-year visual evoked potential and psychophysical serial study. Brain 124:468–479
Henderson APD, Trip SA, Schlottmann PG, Altmann DR, Garway-Heath DF, Plant GT, Miller DH (2008) An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain 131:277–287
Trip SA, Schlottmann PG, Jones SJ, Altmann DR, Garway-Heath DF, Thompson AJ, Plant GP, Miller DH (2005) Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol 58:383–391
Hokazono K, Raza AS, Oyamada MK, Hood DC, Monteiro ML (2013) Pattern electroretinogram in neuromyelitis optica and multiple sclerosis with or without optic neuritis and its correlation with FD-OCT and perimetry. Doc Ophthalmol 127:201–215
Wang J, Cheng H, Hu YS, Tang RA, Frishman LJ (2012) The photopic negative response of the flash electroretinogram in multiple sclerosis. Invest Ophthalmol Vis Sci 53:1315–1323
Klistorner A, Fraser C, Garrick R, Graham S, Arvind H (2008) Correlation between full-field and multifocal VEPs in optic neuritis. Doc Ophthalmol 116:19–27
Fraser CL, Klistorner A, Graham SL, Garrick R, Billson FA, Grigg JR (2006) Multifocal visual evoked potential analysis of inflammatory or demyelinating optic neuritis. Ophthalmology 113:315–323
Yang EB, Hood DC, Rodarte C, Zhang X, Odel JG, Behrens MM (2007) Improvement in conduction velocity after optic neuritis measured with the multifocal VEP. Invest Ophthalmol Vis Sci 48:692–698
Niklas A, Sebraoui H, Heß E, Wagner A, Bergh FT (2009) Outcome measures for trials of remyelinating agents in multiple sclerosis: retrospective longitudinal analysis of visual evoked potential latency. Mult Scler 15:68–74
Holder GE (2006) Chiasmal and retrochiasmal lesions. In: Heckenlively JR, Arden GB (eds) Principles and practice of clinical electrophysiology of vision. MIT Press, Cambridge, pp 857–865
Halliday AM (1993) The visual evoked potential investigation of chiasmal and retrochiasmal lesions, field defects and systemic diseases. In: Halliday AM (ed) Evoked potentials in clinical testing. Churchill Livingstone, Edinburgh, pp 279–357
Brecelj J (1994) Electrodiagnostics of chiasmal compressive lesions. Int J Psychophysiol 16:263–272
Parmar DN, Sofat A, Bowman R, Bartlett JR, Holder GH (2000) Visual prognostic value of the pattern electroretinogram in chiasmal compression. Br J Ophthalmol 84:1024–1026
Brecelj J, Stirn-Kranjc B, Škrbec M (2000) Visual electrophysiology in children with tumours affecting the visual pathway. Doc Ophthamol 101:125–154
Brecelj J (1992) A VEP study of the visual pathway function in compressive lesions of the optic chiasm. Full-field versus half-field stimulation. Electroenceph Clin Neurophysiol 84:209–218
Brecelj J, Denišlič M, Škrbec M (1989) Visual evoked potential abnormalities in chiasmal lesions. Doc Ophthamol 73:139–148
Brecelj J, Denišlič M, Škrbec M (1992) Visual evoked potentials in compressive lesions of the optic chiasm. Neuro-Ophthalmology 12:207–214
Brecelj J, Stirn-Kranjc B (1992) Electropysiologic evaluation of the visual pathway in children. Doc Ophthamol 79:313–323
Štrucl M, Brecelj J, Hawlina M (1997) Visual evoked potential abnormalities in compressive chiasmal lesions: the relevance of central visual field defects. Neuro-Ophthalmology 17:91–100
Mellow TB, Liasis A, Lyons R, Thompson D (2011) When do asymmetrical full-field pattern reversal visual evoked potentials indicate visual pathway dysfunction in children? Doc Ophthalmol 122:9–18
Moradi P, Robson AG, Rose GE, Holder GE (2008) Electrophysiological monitoring in a patient with an optic nerve glioma. Doc Ophthalmol 117:171–174
Mierlo CV, Spileers W, Legius E, Casteels I, Cassiman C (2013) Role of visual evoked potentials in the assessment and management of optic pathway gliomas in children. Doc Ophthamol 127:177–190
Hidajat RR, McLay JL, Goode DH, Hidayat JR (2006) The value of VEP in the diagnosis and post-operative monitoring of meningeoma. Doc Ophthalmol 113:165–169
Danesh-Meyer HV, Carroll SC, Gaskin BJ, Gao A, Gamble GD (2006) Correlation of the multifocal visual evoked potential and standard automated perimetry in compressive optic neuropathies. Invest Ophthalmol Vis Sci 47:1458–1463
Kurent A, Stirn-Kranjc A, Brecelj J (2014) Eletroretinographic characteristics in children with infantile nystagmus syndrome and early onset retinal dystrophies. Eur J Ophthalmol (in press)
Sami DA, Saunders D, Thompson DA, Russell-Eggitt IM, Nischal KK, Jeffery G, Dattani M, Clement RA, Liassis A, Taylor DS (2005) The achiasmia spectrum: congenitally reduced chiasmal decussation. Br J Ophthalmol 89:1311–1317
Thompson DA, Kriss A, Chong K, Harris C, Russell-Eggitt I, Shawat F, Neville BGR, Aclimandos W, Taylor DSI (1999) Visual evoked potential evidence of chiasmal hypoplasia. Ophthalmology 106:2354–2361
Apkarian P, Bour LJ, Barth PG, Wenniger-Prick L, Verbeeten B (1995) Non-decussating retinal-fugal fibre syndrome. An inborn achiasmatic malformation associated with visuotopic misrouting, visual evoked potential ipsilateral asymmetry and nystagmus. Brain 118:1195–1216
Russell-Eggitt I, Kriss A, Taylor DSI (1990) Albinism in childhood: a flash VEP and ERG study. Br J Ophthalmol 74:136–140
Apkarian P, Reits D, Spekreijse H, van Dorp D (1983) A decisive electrophysiological test for human albinism. Electroenceph Clin Neurophysiol 55:513–531
Kriss A, Russell-Eggitt I, Taylor D (1990) Childhood albinism. Visual electrophysiological features. Ophthalmic Paediatr Genet 11:185–192
Kriss A, Russell-Eggitt I, Harris CM, Lloyd IC, Taylor D (1992) Aspects of albinism. Ophthalmic Paediatr Genet 13:89–100
Dorey SE, Neveu MM, Burton LC, Sloper JJ, Holder GE (2003) The clinical features of albinism and their correlation with visual evoked potentials. Br J Ophthalmol 87:767–772
Apkarian P, Bour LJ (2006) Aberrant albino and achiasmat visual pathways: noninvasive electrophysiological assessment. In: Heckenlively JR, Arden GB (eds) Principles and practice of clinical electrophysiology of vision. MIT Press, Cambridge, pp 369–397
Brecelj J, Sustar M, Pečarič-Meglič N, Škrbec M, Stirn-Kranjc B (2012) VEP characteristics in children with achiasmia, in comparison to albino and healthy children. Doc Ophthalmol 124:109–123
Hoffmann MB, Seufert PS, Bach M (2004) Simulated nystagmus suppresses pattern-reversal but not pattern-onset visual evoked potentials. Clin Neurophysiol 115(11):2659–2665
Brecelj J, Stirn-Kranjc B, Pečarič-Meglič N (2012) Achiasmia, electrodiagnosis, and clinical characteristics. In: Harris C, Gottlob I, Sanders J (eds) The challenge of nystagmus: Proceedings of the nystagmus network research workshop, Abingdon, UK, 2–5 September 2009. Nystagmus Network, pp 143–168
Brecelj J, Stirn-Kranjc B, Pečarič-Meglič N, Škrbec M (2007) VEP asymmetry with ophthalmological and MRI findings in two achiasmatic children. Doc Ophthalmol 114:53–65
Brown MC, Southern CL, Anbarasu A, Kaye SB, Fisher AC, Hagan RP, Newman WD (2006) Congenital absence of optic chiasm: demonstration of an uncrossed visual pathway using monocular flash visual evoked potentials. Doc Ophthalmol 113:1–4
Kriss A (1996) Nystagmus and pediatric visual electrophysiology. In: Kimura J, Shibasaki H (eds) Recent advances in clinical neurophysiology: Proceedings of the Xth international congress of EMG and clinical neurophysiology, Kyoto, Japan 15–19 October 1995. Elsevier, Amsterdam, pp 480–487
Pomeranz HD, Agadzi AK, Ekesten B (2006) Achiasmia and unilateral nerve hypoplasia in an otherwise healthy subject. Acta Ophthalmol Scand 84:140–144
Szanyi J, Kubová Z, Kremláček J, Langrová J, Vít F, Kuba M, Szanyi J, Plíšek S (2012) Pattern and motion-related visual-evoked potentials in neuroborreliosis: follow-up study. J Clin Neurophysiol 29:174–180
Tekavčič Pompe M, Stirn Kranjc B, Brecelj J (2014) Chromatic visual evoked potentials in paediatric population. Doc Ophthalmol 128:43–52
Acknowledgments
I am grateful to my mentor professor Tine S Prevec MD, PhD and to Martin A Halliday MD, for their teaching in the field of the Visual Evoked Potentials, and to Anthony Kriss PhD, for his guidance in Paediatric electrophysiology. I am thankful to Maja Šuštar PhD and Barbara Klemenc for preparing figures. This was presented at the Symposium on Clinical Neurophysiology of Vision and on Eye Movements with the 26th Dr. Janez Faganel Memorial Lecture, Ljubljana, 17–18 September 2010. Grant of Slovenian Research Agency (P3-0333).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brecelj, J. Visual electrophysiology in the clinical evaluation of optic neuritis, chiasmal tumours, achiasmia, and ocular albinism: an overview. Doc Ophthalmol 129, 71–84 (2014). https://doi.org/10.1007/s10633-014-9448-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-014-9448-8