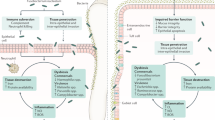
Abstract
Inflammatory bowel disease (IBD) is a group of chronic intestinal inflammatory disorders with a prolonged duration characterized by recurrent relapse and remission. The exact etiology of IBD remains poorly understood despite the identification of relevant risk factors, including individual genetic susceptibility, environmental triggers, and disruption of immune homeostasis. Dysbiosis of the gut microbiota is believed to exacerbate the progression of IBD. Recently, increasing evidence has also linked oral microbiota dysbiosis with the development of IBD. On the one hand, IBD patients show significantly unbalanced composition and function of the oral microbiota known as dysbiosis. On the other, overabundances of oral commensal bacteria with opportunistic pathogenicity have been found in the gut microbiota of IBD patients. Herein, we review the current information on the causative factors of IBD, especially recent evidence of IBD-associated oral microbiota dysbiosis, which has seldom been covered in the previous literature review, highlighting the pathogenic mechanisms of specific oral bacteria in the development of IBD. Ectopic colonization of several oral bacteria, including a subset of Porphyromonas gingivalis, Streptococcus mutans, Fusobacterium nucleatum, Campylobacter concisus, and Klebsiella pneumoniae, may lead to destruction of the intestinal epithelial barrier, excessive secretion of inflammatory cytokines, disruption of the host immune system, and dysbiosis of gut microbiota, consequently aggravating chronic intestinal inflammation. Studying oral microbiota dysbiosis may open future horizons for understanding IBD pathogenesis and provide novel biomarkers for IBD. This review also presents the current treatment and new perspectives for IBD treatment.




Similar content being viewed by others
Abbreviations
- APC:
-
Antigen-presenting cell
- CBP:
-
Collagen-binding protein
- Cc:
-
Campylobacter concisus
- CCK:
-
Cholecystokinin
- CD:
-
Crohn’s disease
- CDI:
-
Clostridioides difficile infection
- CPS:
-
Capsular polysaccharide
- Cr:
-
Campylobacter rectus
- Cu:
-
Campylobacter ureolyticus
- EEN:
-
Exclusive enteral nutrition
- EIMs:
-
Extra-intestinal manifestations
- EPS:
-
Extracellular polymeric substances
- FMT:
-
Fecal microbiota transplant
- Fn:
-
Fusobacterium nucleatum
- GI:
-
Gastrointestinal
- HCs:
-
Healthy controls
- IBD:
-
Inflammatory bowel disease
- IC:
-
Indeterminate colitis
- IECs:
-
Intestinal epithelial cells
- IL:
-
Interleukin
- Kp:
-
Klebsiella pneumoniae
- LPS:
-
Lipopolysaccharides
- MACs:
-
Microbiota-accessible carbohydrates
- MAMP:
-
Microbe-associated molecular pattern
- Pg:
-
Porphyromonas gingivalis
- PN:
-
Parenteral nutrition
- PRR:
-
Pattern recognition receptor
- SCD:
-
Specific carbohydrate diet
- SCFAs:
-
Short-chain fatty acids
- S-ECC:
-
Severe early childhood caries
- Sm:
-
Streptococcus mutans
- TLR:
-
Toll-like receptors
- TNF-α:
-
Tumor necrosis factor alpha
- UC:
-
Ulcerative colitis
- WSD:
-
Westernized diet
- Zot:
-
Zonula occludens toxin
References
Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448:427–434
Ng SC, Shi HY, Hamidi N et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. The Lancet 2017;390:2769–2778
Ma K. Rapid changes in epidemiology of inflammatory bowel disease. Lancet 2017;390:2741–2742
Cho JH, Brant SR. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology 2011;140:1704–1712
Hampe JCA, Croucher PJ, Mirza MM, Mascheretti S, Fisher S. Association between insertion mutation in NOD2 gene and Crohn’s disease in German and British populations. Lancet 2001;357:1925–1928
Kaser A, Lee AH, Franke A. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 2008;134:743–756
Cadwell K, Liu JY, Brown SL et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 2008;456:259–263
Parkes M, Barrett JC, Prescott NJ et al. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat Genet 2007;39:830–832
Barrett JC, Hansoul S, Nicolae DL et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet 2008;40:955–962
Denson LA, Long MD, McGovern DP et al. Challenges in IBD research: update on progress and prioritization of the CCFA’s research agenda. Inflamm Bowel Dis 2013;19:677–682
Satsangi J, Parkes M, Jewell DP. Genetics of ulcerative colitis. The Lancet 1996;348:624–625
Azarschab PPR, Gregor M. Epigenetic control of the E-cadherin gene (CDH1) by CpG methylation in colectomy samples of patients with ulcerative colitis. Genes Chromosom Cancer 2002;35:121–126
Consortium UIG, Barrett JC, Lee JC, et al. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet. 2009;41:1330–1334.
Sano T, Huang W, Hall JA et al. An IL-23R/IL-22 circuit regulates epithelial serum amyloid A to promote local effector Th17 responses. Cell 2016;164:324
Lakatos PLST, Lakatos L. Smoking in inflammatory bowel diseases: good, bad or ugly? World J Gastroenterol 2007;13:6134–6139
Alauzet CMH, Lozniewski A. New insights into Prevotella diversity and medical microbiology. Future Microbiol 2010;5:1695–1718
Hlavaty T, Toth J, Koller T et al. Smoking, breastfeeding, physical inactivity, contact with animals, and size of the family influence the risk of inflammatory bowel disease: a Slovak case-control study. United Eur Gastroenterol J 2013;1:109–119
Luther J, Dave M, Higgins PD, Kao JY. Association between Helicobacter pylori infection and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Inflamm Bowel Dis 2010;16:1077–1084
Lerebours E, Gower-Rousseau C, Merle V et al. Stressful life events as a risk factor for inflammatory bowel disease onset: a population-based case-control study. Am J Gastroenterol 2007;102:122–131
Kronman MP, Zaoutis TE, Haynes K, Feng R, Coffin SE. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics 2012;130:e794-803
Khalili H, Higuchi LM, Ananthakrishnan AN et al. Oral contraceptives, reproductive factors and risk of inflammatory bowel disease. Gut 2013;62:1153–1159
Cipolla G. Nonsteroidal anti-inflammatory drugs and inflammatory bowel disease: current perspectives. Pharmacol Res 2002;46:1–6
Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc 2006;81:1462–1471
Lorenzo D, GianVincenzo Z, Carlo Luca R, et al. Oral-gut microbiota and arthritis: is there an evidence-based axis? J Clin Med. 2019;8:1753. https://doi.org/10.3390/jcm8101753.
Rizzello F, Spisni E, Giovanardi E, et al. Implications of the westernized diet in the onset and progression of IBD. Nutrients. 2019;11:1033
Brown CT, Hug LA, Thomas BC et al. Unusual biology across a group comprising more than 15% of domain Bacteria. Nature 2015;523:208–211
Teigen LM, Geng Z, Sadowsky MJ, et al. Dietary factors in sulfur metabolism and pathogenesis of ulcerative colitis. Nutrients. 2019;11:931
Ananthakrishnan AN, Cagan A, Gainer VS et al. Normalization of plasma 25-hydroxy vitamin D is associated with reduced risk of surgery in Crohn’s disease. Inflamm Bowel Dis 2013;19:1921–1927
Roberts CL, Rushworth SL, Richman E, Rhodes JM. Hypothesis: increased consumption of emulsifiers as an explanation for the rising incidence of Crohn’s disease. J Crohns Colitis 2013;7:338–341
Rodriguez-Palacios A, Harding A, Menghini P et al. The artificial sweetener splenda promotes gut proteobacteria, dysbiosis, and myeloperoxidase reactivity in Crohn’s disease-like ileitis. Inflamm Bowel Dis 2018;24:1005–1020
Sugihara KMM, Nakao M et al. Dietary phosphate exacerbates intestinal inflammation in experimental colitis. J Clin BiochemNutr 2017;61:91–99
Baumgart DC, Sandborn WJ. Crohn’s disease. The Lancet 2012;380:1590–1605
Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. The Lancet 2012;380:1606–1619
Ueno A, Jeffery L, Kobayashi T et al. Th17 plasticity and its relevance to inflammatory bowel disease. J Autoimmun 2018;87:38–49
Brand S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut 2009;58:1152–1167
He J, Li Y, Cao Y, Xue J, Zhou X. The oral microbiome diversity and its relation to human diseases. Folia Microbiol (Praha) 2015;60:69–80
Dewhirst FE, Chen T, Izard J et al. The human oral microbiome. J Bacteriol 2010;192:5002–5017
Zaura E, Keijser BJ, Huse SM, Crielaard W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol 2009;9:259
Nasidze I, Li J, Quinque D, Tang K, Stoneking M. Global diversity in the human salivary microbiome. Genome Res 2009;19:636–643
Bik EM, Long CD, Armitage GC et al. Bacterial diversity in the oral cavity of 10 healthy individuals. ISME J 2010;4:962–974
Xu X, He J, Xue J et al. Oral cavity contains distinct niches with dynamic microbial communities. Environ Microbiol 2015;17:699–710
Wim Crielaard EZ, Annemarie AS, Susan MH, Roy CM, Bart JFK. Exploring the oral microbiota of children at various developmental stages of their dentition in the relation to their oral health. BMC Med Genom 2011;4:22
Dridi B, Raoult D, Drancourt M. Archaea as emerging organisms in complex human microbiomes. Anaerobe 2011;17:56–63
Nguyen-Hieu TKS, Aboudharam G, Drancourt M. Methanogenic archaea in subgingival sites: a review. APMIS 2013;121:467–477
Ghannoum MA, Jurevic RJ, Mukherjee PK et al. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoSPathog 2010;6:e1000713
Qi QGHT, Zhou XD. Frequency, species and molecular characterization of oral Candida in hosts of different age in China. J Oral Pathol Med 2010;34:352–356
Robles-Sikisaka R, Ly M, Boehm T et al. Association between living environment and human oral viral ecology. ISME J 2013;7:1710–1724
Wylie KM, Mihindukulasuriya KA et al. Metagenomic analysis of double-stranded dna viruses in healthy adults. BMC Biol 2014;10:71
Han YW, Wang X. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J Dent Res 2013;92:485–491
Pierer M, Krause C, Häntzschel H. Extraintestinale Manifestationen chronisch-entzündlicher Darmerkrankungen. Z Gastroenterol 2002;40:92–94
Brito F, de Barros FC, Zaltman C et al. Prevalence of periodontitis and DMFT index in patients with Crohn’s disease and ulcerative colitis. J Clin Periodontol 2008;35:555–560
Selwitz RH, Ismail AI, Pitts NB. Dental caries. The Lancet 2007;369:51–59
Kanasi E, Dewhirst FE, Chalmers NI et al. Clonal analysis of the microbiota of severe early childhood caries. Caries Res 2010;44:485–497
Belda-Ferre P, Alcaraz LD, Cabrera-Rubio R et al. The oral metagenome in health and disease. ISME J 2012;6:46–56
Sara SML, Nilminie R et al. Dental caries, prevalence and risk factors in patients with Crohn’s disease. PLoS ONE 2014;9:e91059
Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005;366:1809–1820
Moore LV, Moore WE, Cato EP et al. Bacteriology of human gingivitis. J Dent Res 1987;66:989–995
Vavricka SR, Manser CN, Hediger S et al. Periodontitis and gingivitis in inflammatory bowel disease: a case-control study. Inflamm Bowel Dis 2013;19:2768–2777
Stein JMLF, Zimmer V et al. Clinical periodontal and microbiologic parameters in patients with Crohn’s disease with consideration of the CARD15 genotype. J Periodontol 2010;81:535–545
Docktor MJ, Paster BJ, Abramowicz S et al. Alterations in diversity of the oral microbiome in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2012;18:935–942
Kelsen J, Bittinger K, Pauly-Hubbard H et al. Alterations of the subgingival microbiota in pediatric Crohn’s disease studied longitudinally in discovery and validation cohorts. Inflamm Bowel Dis 2015;21:2797–2805
Said HS, Suda W, Nakagome S et al. Dysbiosis of salivary microbiota in inflammatory bowel disease and its association with oral immunological biomarkers. DNA Res 2014;21:15–25
Zhe XQZ, Tao X, Ning C, Feng C. Dysbiosis and ecotypes of the salivary microbiome associated with inflammatory bowel diseases and the assistance in diagnosis of diseases using oral bacterial profiles. Front Microbiol 2018;9:1136
Qi Y, Zang SQ, Wei J, et al. High-throughput sequencing provides insights into oral microbiota dysbiosis in association with inflammatory bowel disease. Genomics. 2020;113:664–676
Gevers D, Kugathasan S, Denson LA et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014;15:382–392
Qin N, Yang F, Li A et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014;513:59–64
Sears CLG, Microbes WS. Microbiota and colon cancer. Cell Host Microbe 2014;15:317
Zhang LBV, Day AS et al. Isolation and detection of Campylobacter concisus from saliva of healthy individuals and patients with inflammatory bowel disease. J Clin Microbiol 2010;48:2965–2967
Man SM, Zhang L, Day AS et al. Campylobacter concisus and other Campylobacter species in children with newly diagnosed Crohn’s disease. Inflamm Bowel Dis 2010;16:1008–1016
Mukhopadhya ITJM, Hansen R et al. Detection of Campylobacter concisus and other Campylobacter species in colonic biopsies from adults with ulcerative colitis. PLoS ONE 2011;6:e21490
Strauss J, Kaplan GG, Beck PL et al. Invasive potential of gut mucosa-derived Fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm Bowel Dis 2011;17:1971–1978
Höring E, Göpfert G, Schröter G, von Gaisberg U. Frequency and spectrum of microorganisms isolated from biopsy specimens in chronic colitis. Endoscopy 1991;23:325–327
Zhang L, Man SM, Day AS et al. Detection and isolation of campylobacter species other than C. jejuni from children with Crohn’s disease. J Clin Microbiol 2008;47:453–455
Rangan KJH, Howard C. Biochemical mechanisms of pathogen restriction by intestinal bacteria. Trends Biochem Sci 2017;42:887–898
Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol 2013;13:790–801
Johansson MEV, Phillipson M, Petersson J. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Gut Microbes 2010;105:51–54
Pullan RD, Thomas GA, Rhodes M et al. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut 1994;35:353–359
van der Post S, Jabbar KS, Birchenough G et al. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 2019;68:2142–2151
Nakajima M, Arimatsu K, Kato T et al. Oral administration of P. gingivalis induces dysbiosis of gut microbiota and impaired barrier function leading to dissemination of enterobacteria to the liver. PLoS ONE 2015;10:e0134234
Abdi K, Chen T, Klein BA et al. Mechanisms by which Porphyromonas gingivalis evades innate immunity. PLoS ONE 2017;12:e0182164
Jotwani R, Pulendran B, Agrawal S, Cutler CW. Human dendritic cells respond to Porphyromonas gingivalis LPS by promoting a Th2 effector response in vitro. Eur J Immunol 2003;33:2980–2986
Nakano K, Nomura R, Nakagawa I, Hamada S, Ooshima T. Demonstration of Streptococcus mutans with a cell wall polysaccharide specific to a new serotype, k, in the human oral cavity. J Clin Microbiol 2004;42:198–202
Nakano K, Hokamura K, Taniguchi N et al. The collagen-binding protein of Streptococcus mutans is involved in haemorrhagic stroke. Nat Commun 2011;2:485
Kojima A, Nakano K, Wada K et al. Infection of specific strains of Streptococcus mutans, oral bacteria, confers a risk of ulcerative colitis. Sci Rep 2012;2:332
Citron DM. Update on the taxonomy and clinical aspects of the genus fusobacterium. Clin Infect Dis 2002;35:S22
Dharmani P, Strauss J, Ambrose C, Allen-Vercoe E, Chadee K. Fusobacterium nucleatum infection of colonic cells stimulates MUC2 mucin and tumor necrosis factor alpha. Infect Immun 2011;79:2597–2607
Roediger WE. Colonic epithelial metabolism in ulcerative colitis. Gut 1993;34:1646
Tahara T, Shibata T, Kawamura T et al. Fusobacterium detected in colonic biopsy and clinicopathological features of ulcerative colitis in Japan. Dig Dis Sci 2015;60:205–210. https://doi.org/10.1007/s10620-014-3316-y.
Kaplan CW, Ma X, Paranjpe A et al. Fusobacterium nucleatum outer membrane proteins Fap2 and RadD induce cell death in human lymphocytes. Infect Immun 2010;78:4773–4778
Lee SLJ, Ha J et al. Clinical relevance of infections with zoonotic and human oral species of Campylobacter. J Microbiol 2016;54:459–467
Liu F, Lee H, Lan R, Zhang L. Zonula occludens toxins and their prophages in Campylobacter species. Gut Pathog 2016;8:43
Mahendran V, Liu F, Riordan SM et al. Examination of the effects of Campylobacter concisus zonula occludens toxin on intestinal epithelial cells and macrophages. Gut Pathog 2016;8:18
Sørensen NB, Nielsen HL, Varming K, Nielsen H. Neutrophil activation by Campylobacter concisus. Gut pathogens. 2013;5.
Ismail Y, Mahendran V, Octavia S et al. Investigation of the enteric pathogenic potential of oral Campylobacter concisus strains isolated from patients with inflammatory bowel disease. PLoS ONE 2012;7:e38217
Hsu CR, Pan YJ, Liu JY et al. Klebsiella pneumoniae translocates across the intestinal epithelium via Rho GTPase- and phosphatidylinositol 3-kinase/Akt-dependent cell invasion. Infect Immun 2015;83:769–779
Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem 2006;75:39–68
Pan YJ, Lin TL, Hsu CR, Wang JT. Use of a dictyostelium model for isolation of genetic loci associated with phagocytosis and virulence in Klebsiella pneumoniae. Infect Immun 2011;79:997–1006
Atarashi K, Suda W, Luo C et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science 2017;358:359–365
Kitamoto S, Nagao-Kitamoto H, Jiao Y, et al. The intermucosal connection between the mouth and gut in commensal pathobiont-driven colitis. Cell. 2020;182:447–462
Chen W, Chen H, Fu S, et al. Microbiome characterization and re-design by biologic agents for inflammatory bowel disease insights. Bioprocess Biosyst Eng. 2020. https://doi.org/10.1007/s00449-020-02380-y.
Yilmaz B, Juillerat P, Oyas O et al. Microbial network disturbances in relapsing refractory Crohn’s disease. Nat Med 2019;25:323–336
Moayyedi P, Surette MG, Kim PT et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015;149:e106
Paramsothy S, Kamm MA, Kaakoush NO et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. The Lancet. 2017;389:1218–1228
Costello SP, Waters O, Bryant RV et al. Short duration, low intensity, pooled fecal microbiota transplantation induces remission in patients with mild-moderately active ulcerative colitis: a randomised controlled trial. Gastroenterology 2017;152:S198–S199
Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Science 2018;362:776–780
Grover Z, Muir R, Lewindon P. Exclusive enteral nutrition induces early clinical, mucosal and transmural remission in paediatric Crohn’s disease. J Gastroenterol 2014;49:638–645
Suskind DL, Wahbeh G, Cohen SA et al. Patients perceive clinical benefit with the specific carbohydrate diet for inflammatory bowel disease. Dig Dis Sci 2016;61:3255–3260. https://doi.org/10.1007/s10620-016-4307-y.
Elson CO, Layden TJ, Nemchausky BA et al. An evaluation of total parenteral nutrition in the management of inflammatory bowel disease. Dig Dis Sci 1980;25:42–48. https://doi.org/10.1007/BF01312731.
Acknowledgments
We acknowledge Prof. Yonghe Luo at Jinling Hospital and Xuwen Liu at Middlebury Institute of International Studies at Monterey for their linguistic assistance.
Funding
This review was funded by the National Natural Science Foundation of China, grant nos. 81873559 and 81570506.
Author information
Authors and Affiliations
Contributions
Conceptualization, FYW and YQ; writing: original draft preparation and figure design, YQ; writing: review and editing, HMW, ZY, and YFZ; revision, LJ and MFY; final revision: FYW. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qi, Y., Wu, Hm., Yang, Z. et al. New Insights into the Role of Oral Microbiota Dysbiosis in the Pathogenesis of Inflammatory Bowel Disease. Dig Dis Sci 67, 42–55 (2022). https://doi.org/10.1007/s10620-021-06837-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-06837-2