Abstract
Background
We previously showed that Lactobacillus brevis-derived polyphosphate (poly P) exerts a curative effect on intestinal inflammation. However, whether or not poly P improves the inflammation and injury of distant organs remains unclear.
Aims
We aimed to investigate the change in the intestinal microbiome and to evaluate the protective effect of poly P on injuries in a cerulein-induced acute pancreatitis (AP) mouse.
Methods
Poly P was orally administered to BALB/C mice every day for 24 days, and then mice were intraperitoneally injected with cerulein. Before cerulein injection, stool samples were collected and analyzed by 16S rRNA gene sequencing. Mice were sacrificed at 24 h after the last cerulein injection; subsequently, the serum, pancreas, and colon were collected.
Results
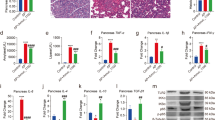
The microbial profile differed markedly between poly P and control group. Notably, the levels of beneficial bacteria, including Alistipes and Candidatus_Saccharimonas, were significantly increased, while those of the virulent bacteria Desulfovibrio were decreased in the poly P group. The elevations of the serum amylase and lipase levels by cerulein treatment were suppressed by the pre-administration of poly P for 24 days, but not for 7 days. The numbers of cells MPO-positive by immunohistology were decreased and the levels of MCP-1 significantly reduced in the AP + Poly P group. An immunofluorescence analysis showed that the ZO-1 and occludin in the colon was strongly augmented in the epithelial cell membrane layer in the AP + Poly P group.
Conclusions
Poly P attenuates AP through both modification of the intestinal microbiome and enhancement of the intestinal barrier integrity.







Similar content being viewed by others
References
Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med.. 2018;24:392–400.
Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology.. 2013;145:946–953.
Chen J, Huang C, Wang J, et al. Dysbiosis of intestinal microbiota and decrease in paneth cell antimicrobial peptide level during acute necrotizing pancreatitis in rats. PLoS ONE.. 2017;12:e0176583.
Huang C, Chen J, Wang J, et al. Dysbiosis of intestinal microbiota and decreased antimicrobial peptide level in Paneth cells during hypertriglyceridemia-related acute necrotizing pancreatitis in rats. Front Microbiol.. 2017;8:776.
FAO/WHO. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. 2001.
Segawa S, Fujiya M, Konishi H, et al. Probiotic-derived polyphosphate enhances the epithelial barrier function and maintains intestinal homeostasis through integrin-p38 MAPK pathway. PLoS ONE. 2011;6:e23278.
Kornberg A, Rao NN, Ault-Riche D. Inorganic polyphosphate: a molecule of many functions. Annu Rev Biochem.. 1999;68:89–125.
Tanaka K, Fujiya M, Konishi H, et al. Probiotic-derived polyphosphate improves the intestinal barrier function through the caveolin-dependent endocytic pathway. Biochem Biophys Res Commun.. 2015;467:541–548.
Kashima S, Fujiya M, Konishi H, et al. Polyphosphate, an active molecule derived from probiotic Lactobacillus brevis, improves the fibrosis in murine colitis. Transl Res.. 2015;166:163–175.
Fujiya M, Ueno N, Kashima S, et al. Long-Chain polyphosphate is a potential agent for inducing mucosal healing of the colon in ulcerative Colitis. Clin Pharmacol Ther.. 2020;107:452–461.
Algul H, Tando Y, Schneider G, Weidenbach H, Adler G, Schmid RM. Acute experimental pancreatitis and NF-kappaB/Rel activation. Pancreatology.. 2002;2:503–509.
Zhu Y, He C, Li X, et al. Gut microbiota dysbiosis worsens the severity of acute pancreatitis in patients and mice. J Gastroenterol.. 2019;54:347–358.
Watanabe T, Kudo M, Strober W. Immunopathogenesis of pancreatitis. Mucosal Immunol.. 2017;10:283–298.
Maier SK, Scherer S, Loessner MJ. Long-chain polyphosphate causes cell lysis and inhibits Bacillus cereus septum formation, which is dependent on divalent cations Appl Environ Microbiol. 1999;65:3942-3949; Lee RM, Hartman PA, Stahr HM, Olson DG, Williams FD. Antibacterial Mechanism of Long-Chain Polyphosphates in Staphylococcus aureus J Food Prot. 1994;57:289-294.
Hasebe T, Ueno N, Musch MW, et al. Daikenchuto (TU-100) shapes gut microbiota architecture and increases the production of ginsenoside metabolite compound K. Pharmacol Res Perspect.. 2016;4:e00215.
Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods.. 2010;7:335–336.
Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol.. 2011;12:R60.
Yang ZW, Meng XX, Xu P. Central role of neutrophil in the pathogenesis of severe acute pancreatitis. J Cell Mol Med.. 2015;19:2513–2520.
Rychter JW, van Minnen LP, Verheem A, et al. Pretreatment but not treatment with probiotics abolishes mouse intestinal barrier dysfunction in acute pancreatitis. Surgery.. 2009;145:157–167.
van Minnen LP, Timmerman HM, Lutgendorff F, et al. Modification of intestinal flora with multispecies probiotics reduces bacterial translocation and improves clinical course in a rat model of acute pancreatitis. Surgery.. 2007;141:470–480.
Muftuoglu MAT, Isikgor S, Tosun S, Saglam A. Effects of probiotics on the severity of experimental acute pancreatitis. Eur J Clin Nutrit.. 2005;60:464–468.
Jin D-Y, Lutgendorff F, Nijmeijer RMet al.. Probiotics Prevent Intestinal Barrier Dysfunction in Acute Pancreatitis in Rats via Induction of Ileal Mucosal Glutathione Biosynthesis PLoS ONE. 2009;4.
Jo IJ, Bae GS, Choi SBet al.. Fisetin attenuates cerulein-induced acute pancreatitis through down regulation of JNK and NF-kappaB signaling pathways Eur J Pharmacol. 2014;737:149-158; Ren Z, Li H, Zhang Met al.. A Novel Derivative of the Natural Product Danshensu Suppresses Inflammatory Responses to Alleviate Caerulein-Induced Acute Pancreatitis Front Immunol. 2018;9:2513.
Zhang H, Yang W, Li Y, et al. Astaxanthin ameliorates cerulein-induced acute pancreatitis in mice. Int Immunopharmacol.. 2018;56:18–28.
Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc National Acad Sci United States America.. 2007;104:13780–13785.
Wu M, Li P, An Y, et al. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol Res.. 2019;150:104489.
Huang Y, Li M, Zhou L, et al. Effects of Qingluo Tongbi decoction on gut flora of rats with adjuvant-induced arthritis and the underlying mechanism evid based complement. Alternat Med.. 2019;2019:6308021.
Rowan F, Docherty NG, Murphy M, Murphy B, Calvin Coffey J, O’Connell PR. Desulfovibrio bacterial species are increased in ulcerative colitis. Diseases Colon Rectum.. 2010;53:1530–1536.
Sawin EA, De Wolfe TJ, Aktas B, et al. Glycomacropeptide is a prebiotic that reduces Desulfovibrio bacteria, increases cecal short-chain fatty acids, and is anti-inflammatory in mice. Am J Physiol Gastrointest Liver Physiol.. 2015;309:G590–G601.
Roediger WE, Moore J, Babidge W. Colonic sulfide in pathogenesis and treatment of ulcerative colitis. Dig Dis Sci.. 1997;42:1571–1579. https://doi.org/10.1023/A:1018851723920.
Hagiya H, Kimura K, Nishi I, et al. Desulfovibrio desulfuricans bacteremia: A case report and literature review. Anaerobe.. 2018;49:112–115.
Zhang J, Yu WQ, Wei T, et al. Effects of short-peptide-based enteral nutrition on the intestinal microcirculation and mucosal barrier in mice with severe acute pancreatitis. Mol Nutr Food Res.. 2020;64:e1901191.
Jia L, Chen H, Yang J, et al. Combinatory antibiotic treatment protects against experimental acute pancreatitis by suppressing gut bacterial translocation to pancreas and inhibiting NLRP3 inflammasome pathway. Innate Immun.. 2020;26:48–61.
Ryan CM, Schmidt J, Lewandrowski K, et al. Gut macromolecular permeability in pancreatitis correlates with severity of disease in rats. Gastroenterology.. 1993;104:890–895.
Tsuji Y, Watanabe T, Kudo M, Arai H, Strober W, Chiba T. Sensing of commensal organisms by the intracellular sensor NOD1 mediates experimental pancreatitis. Immunity.. 2012;37:326–338.
Rau B, Baumgart K, Kruger CM, Schilling M, Beger HG. CC-chemokine activation in acute pancreatitis: enhanced release of monocyte chemoattractant protein-1 in patients with local and systemic complications. Intensive Care Med.. 2003;29:622–629.
Ishibashi T, Zhao H, Kawabe K, et al. Blocking of monocyte chemoattractant protein-1 (MCP-1) activity attenuates the severity of acute pancreatitis in rats. J Gastroenterol.. 2008;43:79–85.
Bhatia M, Ramnath RD, Chevali L, Guglielmotti A. Treatment with bindarit, a blocker of MCP-1 synthesis, protects mice against acute pancreatitis. Am J Physiol Gastrointest Liver Physiol.. 2005;288:G1259–G1265.
Frossard JL, Lenglet S, Montecucco F, et al. Role of CCL-2, CCR-2 and CCR-4 in cerulein-induced acute pancreatitis and pancreatitis-associated lung injury. J Clin Pathol.. 2011;64:387–393.
Bedrosian AS, Nguyen AH, Hackman M, et al. Dendritic cells promote pancreatic viability in mice with acute pancreatitis. Gastroenterology.. 2011;141:e1911–e1914.
Shrivastava P, Bhatia M. Essential role of monocytes and macrophages in the progression of acute pancreatitis. World J Gastroenterol.. 2010;16:3995–4002.
Tsai MJ, Chen C, Chen SH, Huang YT, Chiu TH. Pomalidomide suppresses cerulein-induced acute pancreatitis in mice. J Gastroenterol.. 2011;46:822–833.
Ding SP, Li JC, Jin C. A mouse model of severe acute pancreatitis induced with caerulein and lipopolysaccharide. World J Gastroenterol.. 2003;9:584–589.
Acknowledgments
We thank Akemi Kita and Chikage Yamamura for their technical assistance.
Funding
This paper was supported by Grants-in-Aid for Scientific Research (C), No. 18K08906 (S. Takauji), the Akiyama Life Science Foundation (2019). This study was partially supported by Grants-in-Aid for Scientific Research, No. 26460956 (M. Fujiya), Translational Research Network Program of Japan Agency for Medical Research and Development, No. C40 (M. Fujiya) and Development and Intractable Disease Health, and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare.
Author information
Authors and Affiliations
Contributions
S.T., H.K. and M.F. conceived and planned the experiments, wrote the manuscript and supervised all investigations. S.T. and H.K. carried out the experiments. N.U., H.T., H.S., and S.I. helped design the studies and interpret the data. S.K., Y.M., K.M., and K.O. supervised the findings of this work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Fujiya reports grants from Japanese Grants-in-Aid for Scientific Research, grants from Translational Research Network Program of Japan Agency for Medical Research and Development, non-financial support from Development and Intractable Disease Health, and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare. During the conduct of the study, he reports grants and personal fees from Yakult Honsha Co., Ltd., grants and personal fees from Nippon Kayaku Co., Ltd., grants, personal fees and non-financial support from EA Pharma Co., Ltd., personal fees from Novartis Pharmaceuticals, grants from Boehringer Ingelheim GmbH, personal fees from Technical Information Institute Co., Ltd., personal fees from Pfizer Inc., grants and personal fees from Takeda Pharmaceutical Company Limited, grants and personal fees from Daiichi Sankyo Company, Limited, grants from Shionogi & Co., Ltd., personal fees from Olympus Co., Ltd., personal fees from Janssen Pharmaceutical K.K., personal fees from Kyorin Pharmaceutical Co., Ltd., personal fees from Taisho Toyama Pharmaceutical Co., Ltd., grants from AstraZeneca, personal fees from Tomakomai Minpo Co., Ltd., personal fees from Meiji Seika Pharma Co., Ltd., grants and personal fees from Mochida Pharmaceutical Co., Ltd., grants from Astellas Pharma Inc., personal fees from Mitsubishi Tanabe Pharma Corporation, grants from Baxter International Inc, personal fees from Bristol-Myers Company, grants and personal fees from Chugai Pharmaceutical Co., Ltd., grants from MSD K.K., grants and personal fees from Otsuka Pharmaceutical Co., Ltd., grants and personal fees from Kyowa Hakko Kirin Co., Ltd., grants from Taiho Pharmaceutical Co., Ltd., personal fees from Zeria Pharmaceutical Co., Ltd., grants from Alexion Pharmaceutical Co., Ltd., grants, personal fees and non-financial support from Ajinomoto Pharmaceutical Co., Ltd., personal fees from Asahi Kasei Corporation, grants and non-financial support from Sapporo Breweries Ltd., grants and personal fees from Eisai Co., Ltd., grants from GlaxoSmithKline K.K., grants from AbbVie Inc, grants from Sumitomo Dainippon Pharma Co., Ltd., grants from Asuka Pharmaceutical Co., Ltd., grants from Boston Scientific Corporation or its affiliates, and grants and non-financial support from Kamui Pharma. Inc. outside the submitted work. In addition, Dr. Fujiya has been issued patents for intestinal protectants (JP 5660508 and EP 2559437).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An editorial commenting on this article is available at https://doi.org/10.1007/s10620-020-06809-y.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takauji, S., Konishi, H., Fujiya, M. et al. Polyphosphate, Derived from Lactobacillus brevis, Modulates the Intestinal Microbiome and Attenuates Acute Pancreatitis. Dig Dis Sci 66, 3872–3884 (2021). https://doi.org/10.1007/s10620-020-06747-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06747-9