Abstract
Aim
To observe the therapeutic effect of low-dose amitriptyline (AMT) on epigastric pain syndrome (EPS) in patients with functional dyspepsia.
Methods
Sixty patients with EPS were randomly divided into the following two groups for a four-week clinical trial: routine treatment with pantoprazole (RT group) and the AMT group. The RT group was treated with 40 mg of pantoprazole once daily. The AMT group received 25 mg of AMT once daily before bedtime. The Nepean Dyspepsia Index (NDI) checklist, Hamilton Rating Scale of Anxiety/Depression (HAMA/HAMD), and Pittsburgh Sleep Quality Index (PSQI) were employed to evaluate dyspepsia symptoms, psychological distress, and sleep, respectively.
Results
All items were similar between the two groups before treatment (0 week). After 4 weeks of treatment, the NDI–symptom checklist score as well as the severity and bothersomeness of EPS in the AMT group was significantly decreased compared with those in the RT group (p < 0.05). However, no differences were found in the frequency of NDI checklist, psychological status (HAMD/HAMA scores) of EPS, or sleep quality (PSQI score) between the two groups after treatment. In addition, the time to fall asleep was shorter in the AMT group compared with the RT group after 4 weeks of treatment (p < 0.05).
Conclusion
Low-dose AMT effectively improved the dyspepsia symptoms and the time to fall asleep in the EPS patients, compared with pantoprazole, although it did not reduce the psychological distress. Therefore, AMT could be considered as a good candidate for EPS treatment in the clinic.
Similar content being viewed by others
Introduction
Functional dyspepsia (FD) is one of the most common functional gastrointestinal disorders (FGIDs) [1]. In an investigation of 321,000 individuals, the prevalence of FD was found to be 21%. The risk of FD is elevated in smokers, those who are infected with Helicobacter pylori, as well as those who have taken nonsteroidal anti-inflammatory drugs [2]. The life quality and work efficiency of FD patients are significantly affected, and it also increases the cost of health care [3,4,5]. In 2016, the Rome criteria of FD diagnosis were modified to the Rome IV criteria [6]. FD remains as an umbrella term referring to patients who suffer from epigastric pain syndrome (EPS) and postprandial distress syndrome (PDS). The principal symptoms for EPS are bothersome epigastric pain and burning, and one or both of the symptoms present at least once weekly. Those symptoms are related to visceral hypersensitivity, infection by Helicobacter pylori, and acid exposure. Empirically, a conventional dose of a proton pump inhibitors (PPI) is usually applied to treat these patients. Tricyclic agents (as Amitriptyline) are often used to treat the abdominal pain symptoms of FD patients [7, 8].
FD is one of the disorders of gut–brain interaction, and the brain–gut connection explains psychological factor is linked so close to gastrointestinal symptoms [6, 9]. More and more clinicians recognize the value and importance of antidepressants in treating patients with FD. Actually, antidepressants have been successfully introduced to treat cyclic vomiting syndrome and irritable bowel syndrome [10, 11]. These findings encouraged clinicians to widely apply antidepressants for the management of FD. Amitriptyline (AMT) is a tricyclic antidepressant; however, its application is limited clinically because of its increased risk of adverse cardiac events at high doses (more than 100 mg/d) [12]. Nevertheless, the fact that low-dose AMT is typically used in the treatment of FGIDs may limit the risk of side effect [13,14,15]. The mechanisms of amitriptyline action in FGIDs are unknown, which may be connected with a change in the gastrointestinal motility and sensitivity by central neuromodulators (5-hydroxytryptamine and noradrenalin) [9]. To the best of our knowledge, few studies in the literature have focused on the application of low-dose AMT for the treatment of EPS. We hypothesized that low-dose of AMT could be improved the dyspepsia symptoms and reduced the psychological distress in the treatment of EPS. Therefore, the purpose of this study was to explore the application of low-dose AMT to treat EPS patients.
Materials and Methods
Ethics Authorization
This clinical study was a prospective, randomized, controlled trial for EPS patients and was approved by the Ethics Committee of Guangzhou First People’s Hospital. This study was registered in the Chinese Clinical Trial Registry Center (Registration Number: ChiCTR-TRC-12001968). All patients provided a written informed consent.
Patients
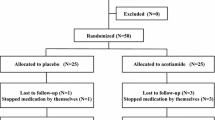
Sixty EPS patients were enrolled from September 2016 to June 2018 at the Gastroenterology Outpatient Clinic of Guangzhou Nansha Central Hospital. All the patients were screened by gastroenterologists in our team and were considered eligible to participate in the enrollment.
Patients fulfilled the criteria: (1) diagnosed with EPS according to Rome IV [6]; (2) aged 18 to 65; and (3) willing to participate in the study. The exclusion criteria were as follows: (1) younger than 18 years old and older than 65 years old; (2) pregnant or breastfeeding; (3) a history of abdominal surgery; (4) a history of hepatic, renal, or metabolic disorders; (5) allergic to AMT or pantoprazole; (6) contraindications of AMT, such as serious heart disease, hypertension, prostatic disease, glaucoma, history of seizure, retention of urine, bronchial asthma, and abnormal thyroid disease; and (7) use of similar drugs during the last 2 weeks.
Grouping
All the eligible patients were randomly divided into the AMT group and the routine treatment (RT) group by an independent member in our team using a computer-generated random numbers table, each of which had 30 patients. The RT group was treated with 40 mg of pantoprazole once daily. The AMT group received 25 mg of AMT once daily before bedtime. The observation and treatment period was 4 weeks.
Measurement
A series of questionnaires including the Nepean Dyspepsia Index (NDI)–symptom checklist, Hamilton Rating Scale of Anxiety/Depression (HAMA/HAMD), and Pittsburgh Sleep Quality Index (PSQI) were completed by the patients before treatment and at 4 weeks after treatment. The NDI [16] checklist questionnaire is a disease-specific index for dyspepsia, which investigates the severity, frequency, and bothersomeness of fifteen gastrointestinal symptoms in the past 2 weeks. High scores indicate severe symptoms. HAMA [17] is applied to measure the degree of anxiety; a higher score suggests worse anxiety. HAMD [18] is used to assess the extent of depression; a higher score indicates a more serious state of depression. PSQI [19] is applied to evaluate sleep quality and disturbances during the last month; a higher score indicates worse sleep quality. Treatment response was considered when there was more than a 50% decrease in the NDI–symptom checklist score [20]. The response was determined as follows: [(score after 4 weeks of treatment—score before treatment)/score before treatment] × 100. The treatment response index of the two groups was calculated independently.
Statistical Analysis
All data were analyzed with SPSS software. Continuous data were presented as the mean ± standard deviation, whereas count data were expressed as frequencies or numbers. Continuous data of the comparisons between the two groups were treated by the t test, and the Chi-square test was employed for count data analysis. In our study, P < 0.05 was considered as statistically significant.
Results
General Information
Of the 60 participants with EPS, 5 (3 in the AMT group and 2 in the RT group) were lost to follow-up. Two patients dropped out from AMT group because of unbearable sleepiness, and the other one dropped out due to traveling abroad. Two patients dropped out from RT group due to a lack of efficacy. Therefore, 27 EPS patients in the AMT group and 28 EPS patients in the RT group were finally included in this study. The average age (years), height (m), weight (kg), and sex of each group are listed in Table 1. There were no significant differences in terms of sex, age, height, or weight between the two groups.
Improvement of Dyspepsia Symptoms and Treatment Efficacy
The baseline data (0 weeks) of the NDI–symptom checklist score, frequency, severity, and bothersomeness were similar between the AMT and RT groups (all P > 0.05, Table 2). After 4 weeks of treatment, the NDI–symptom checklist score of the AMT group was significantly lower than that of the RT group (P < 0.05, Table 2). The severity and bothersomeness of EPS in the AMT group were significantly lower than those in the RT group (P < 0.05, respectively, Table 2). However, there was no difference in the frequency of EPS between the two groups (P > 0.05, Table 2).
After 4 weeks of therapy, the treatment efficacy between the two groups was evaluated by the response rate. The response rate of the AMT group was significantly greater than that of the RT group (62.96% vs. 28.57%, P = 0.010).
Alteration of Psychological Scores After Treatment
Before treatment, both the HAMD and HAMA scores of the AMT and RT groups were similar (P > 0.05, Table 2). After 4 weeks of treatment, no significant differences in the psychological measures between the AMT and RT groups were found (P > 0.05, Table 2).
Alterations of PSQI After Treatment
The PSQI scores were not significantly different between the AMT and RT groups before and after treatment (P > 0.05, Table 2). However, the time to fall asleep in the AMT group was significantly shorter than that in the RT group after treatment (P < 0.05, Table 2).
Adverse Effects
Various adverse effects were recorded in the AMT group, including sleepiness (14.8%), dry mouth (11.1%), and constipation (7.4%). No cardiac events were reported. The adverse effects could be tolerant and improved within 1 week without treatment. There were no obvious adverse effects in the RT group.
Discussion
As the etiology of FD is multifactorial, PPIs, prokinetic drugs, and antidepressants are usually used for the management of FD. Evidence supporting these approaches for FD treatment is still unclear and inconsistent [21,22,23,24,25,26]. According to the Rome IV criteria, FD is subdivided into two subgroups: EPS and PDS. These two clinical types have different pathogeneses. Visceral hypersensitivity is believed to play an important role in the pathophysiology of EPS [27, 28]. Pantoprazole is a PPI drug that inhibits the final step in gastric acid production; therefore, it is widely applied for the treatment of erosive esophagitis associated with gastroesophageal reflux disorders as well as pathological hypersecretory diseases. It is also a conventional treatment for EPS [29]. In clinical practice, there are no very effective therapeutic approaches for EPS, due to complex etiology and recurrent characteristics of the disease, and lack of validated end points. Antidepressants are often used to treat those patients [30]. In this study, one of these, AMT was employed to treat EPS patients.
AMT belongs to the group of tricyclic antidepressants, a high dose (100–250 mg/d) of which can cause serious side effects. However, low-dose AMT is reported to be well tolerated and has been applied to treat patients with FD, irritable bowel syndrome, and Globus pharynges, especially for the improvement of abdominal pain [8, 15, 31,32,33]. Namin et al. have studied 31 patients with cyclic vomiting syndrome, another functional gastrointestinal disorder affected by psychological factors. They applied low-dose AMT (1 mg/kg/day, equivalent to 60 mg/day) to treat cyclic vomiting syndrome patients; their results showed a 93% efficacy rate of symptom relief, and 26% of them achieved full remission [10]. In this study, we used a much lower dose of AMT (25 mg/day) to treat EPS, compared with the study by Namin et al. A randomized, placebo-controlled, double-blind, two-period cross-over trial reported that low-dose AMT could decrease visceral sensitivity in healthy Chinese volunteers [34]. In addition, Thoua et al. [15] have reported that irritable bowel syndrome patients treated with AMT (25–50 mg/d) for 3 months had an obviously decreased rectal hypersensitivity to electrical stress. Moreover, a multicenter, double-blind trial demonstrated that low-dose AMT was beneficial for some patients with FD, particularly those with ulcer-like FD [35]. In this study, we carried out a prospective, randomized, and controlled trial to explore the efficacy of low-dose AMT (25 mg/day) to treat EPS. The treatment efficiency of the EPS patients with AMT was extremely greater than that with RT treatment. We found that low-dose AMT could obviously improve the dyspepsia symptoms and the time to fall asleep in the EPS patients, yet it did not reduce the psychological distress. As expected, the adverse reactions to low-dose AMT were mild and tolerant. These responses may be related to the brain–gut axis regulation by inhibition of the norepinephrine transporter and serotonin transporter to alter neurotransmitter systems of pain centers in the brain.
However, some limitations of this study must be acknowledged. First, the sample size of the EPS patients was not large enough. Future studies including larger sample sizes are required. Second, EPS diagnosis was only based on the symptomatic pattern, and no objective indices were available for EPS diagnosis. Finally, we were not able to distinguish whether the shorter time to fall asleep was caused by dyspepsia symptom relief or whether the dyspepsia symptom relief was induced by the improvement in the time to fall asleep. For this point, we will design some rigorous trials in the future to explore the cause and effect.
In conclusion, low-dose AMT was demonstrated to be efficacious to relieve dyspepsia symptoms and to shorten the time to fall asleep in EPS patients, although it did not reduce the psychological distress. The results of this study provide another effective method to relieve dyspepsia symptoms in the clinic and to improve the time to fall asleep for EPS patients. Therefore, it could be considered as a good candidate for EPS treatment in the clinic.
Abbreviations
- AMT:
-
Amitriptyline
- EPS:
-
Epigastric pain syndrome
- FD:
-
Functional dyspepsia
- FGIDs:
-
Functional gastrointestinal disorders
- HAMA:
-
Hamilton Rating Scale of Anxiety
- HAMD:
-
Hamilton Rating Scale of Depression
- NDI:
-
Nepean Dyspepsia Index
- PDS:
-
Postprandial distress syndrome
- PPIs:
-
Proton pump inhibitors
- RT:
-
Routine treatment
References
Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479.
Ford AC, Marwaha A, Sood R, et al. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut.. 2015;64:1049–1057.
Brook RA, Kleinman NL, Choung RS, et al. Functional dyspepsia impacts absenteeism and direct and indirect costs. Clin Gastroenterol Hepatol. 2010;8:498–503.
Talley NJ. Functional gastrointestinal disorders as a public health problem. Neurogastroenterol Motil. 2008;20(Suppl 1):121–129.
Aro P, Talley NJ, Agréus L, et al. Functional dyspepsia impairs quality of life in the adult population. Aliment Pharmacol Ther. 2011;33:1215–1224.
Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016;150:1262–1279.
Otaka M, Jin M, Odashima M, et al. New strategy of therapy for functional dyspepsia using famotidine, mosapride and amitriptyline. Aliment Pharmacol Ther. 2005;21(Suppl 2):42–46.
Mertz H, Fass R, Kodner A, et al. Effect of amitriptyline on symptoms, sleep, and visceral perception in patients with functional dyspepsia. Am J Gastroenterol.. 1998;93:160–165.
Drossman DA, Tack J, Ford AC, et al. Neuromodulators for functional gastrointestinal disorders (Disorders of Gut-Brain Interaction): a Rome foundation working team report. Gastroenterology. 2018;154:1140–1171.
Namin F, Patel J, Lin Z, et al. Clinical, psychiatric and manometric profile of cyclic vomiting syndrome in adults and response to tricyclic therapy. Neurogastroenterol Motil. 2007;19:196–202.
Ford AC, Quigley EM, Lacy BE, et al. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1350–1365.
Ray WA, Meredith S, Thapa PB, et al. Cyclic antidepressants and the risk of sudden cardiac death. Clin Pharmacol Ther. 2004;75:234–241.
Törnblom H, Drossman DA. Centrally targeted pharmacotherapy for chronic abdominal pain. Neurogastroenterol Motil. 2015;27:455–467.
Teitelbaum JE, Arora R. Long-term efficacy of low-dose tricyclic antidepressants for children with functional gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2011;53:260–264.
Thoua NM, Murray CD, Winchester WJ, et al. Amitriptyline modifies the visceral hypersensitivity response to acute stress in the irritable bowel syndrome. Aliment Pharmacol Ther. 2009;29:552–560.
Talley NJ, Haque M, Wyeth JW, et al. Development of a new dyspepsia impact scale: the Nepean Dyspepsia Index. Aliment Pharmacol Ther. 1999;13:225–235.
Matza LS, Morlock R, Sexton C, et al. Identifying HAM-A cutoffs for mild, moderate, and severe generalized anxiety disorder. Int J Methods Psychiatr Res. 2010;19:223–232.
Calotă DR, Niţescu C, Marinescu S, et al. Correlations between morphological appearance and psychosocial difficulties in patients with extensive burns who received allotransplant. Rom J Morphol Embryol. 2012;53:703–711.
Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213.
Jiang SM, Jia L, Liu J, et al. Beneficial effects of antidepressant mirtazapine in functional dyspepsia patients with weight loss. World J Gastroenterol.. 2016;22:5260–5266.
Leung WK, Wu JC, Chan FK, et al. Initial treatment with lansoprazole in young dyspeptic patients with negative urea breath test result: a randomized controlled trial with 12-month follow-up. Am J Gastroenterol. 2007;102:1483–1488.
Wang WH, Huang JQ, Zheng GF, et al. Effects of proton-pump inhibitors on functional dyspepsia: a meta-analysis of randomized placebo-controlled trials. Clin Gastroenterol Hepatol. 2007;5:178–185.
Tack J, Masclee A, Heading R, et al. A dose-ranging, placebo-controlled, pilot trial of Acotiamide in patients with functional dyspepsia. Neurogastroenterol Motil. 2009;21:272–280.
Matsueda K, Hongo M, Tack J, et al. Clinical trial: dose-dependent therapeutic efficacy of acotiamide hydrochloride (Z-338) in patients with functional dyspepsia—100 mg t.i.d is an optimal dosage. Neurogastroenterol Motil. 2010;22:618.
Dinan TG, Mahmud N, Rathore O, et al. A double-blind placebo-controlled study of buspirone-stimulated prolactin release in non-ulcer dyspepsia: are central serotoninergic responses enhanced? Aliment Pharmacol Ther. 2001;15:1613–1618.
Van Kerkhoven LA, Laheij RJ, Aparicio N, et al. Effect of the antidepressant venlafaxine in functional dyspepsia: a randomized, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2008;6:746–752.
Mimidis K, Tack J. Pathogenesis of dyspepsia. Dig Dis. 2008;26:194–202.
Sarnelli G, Cuomo R, Janssens J, et al. Symptom patterns and pathophysiological mechanisms in dyspeptic patients with and without Helicobacter pylori. Dig Dis Sci. 2003;48:2229–2236.
Madisch A, Andresen V, Enck P, et al. The diagnosis and treatment of functional dyspepsia. Dtsch Arztebl Int. 2018;115(13):222–232.
Vandenberghe A, Schol J, Van den Houte K, et al. Current and emerging therapeutic options for the management of functional dyspepsia. Expert Opin Pharmacother. 2020;3:1–12.
Vahedi H, Merat S, Momtahen S, et al. Clinical trial: the effect of amitriptyline in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;27:678–684.
Braak B, Klooker TK, Wouters MM, et al. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2011;34:638–648.
You LQ, Liu J, Jia L, et al. Effect of low-dose amitriptyline on globus pharyngeus and its side effects. World J Gastroenterol. 2013;19:7455–7460.
Huang W, Jiang SM, Jia L, et al. Effect of amitriptyline on gastrointestinal function and brain-gut peptides: a double-blind trial. World J Gastroenterol. 2013;19:4214–4220.
Talley Nicholas J, Richard Locke G, et al. Effect of amitriptyline and escitalopram on Functional Dyspepsia: a multi-center, randomized, controlled study. Gastroenterology. 2015;149(2):340–349.
Acknowledgments
We would like to thank Yao-xing Huang for his contributions in collecting cases.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Liu, J., Jia, L., Jiang, Sm. et al. Effects of Low-Dose Amitriptyline on Epigastric Pain Syndrome in Functional Dyspepsia Patients. Dig Dis Sci 66, 521–525 (2021). https://doi.org/10.1007/s10620-020-06191-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06191-9