Abstract
Background
Environmental enteropathy (EE) is associated with stunting, impairment of responses to oral vaccines, and other adverse health consequences in young children throughout the developing world. EE is characterized by chronic low-grade intestinal inflammation and disrupted epithelial barrier integrity, partly resulting from dysregulation of tight junction proteins, observed in other enteropathies such as celiac disease. During EE, this dysregulation of tight junction expression amplifies translocation of pathogenic bacteria across the intestinal mucosa.
Aims
The aim was to determine whether enteropathogen-mediated epithelial barrier failure can be ameliorated using contra-pathogenicity therapies.
Methods
Intestinal epithelial barrier damage was assessed in Caco-2 cells incubated with three important enteropathogens identified in EE patients: Enteropathogenic Escherichia coli (EPEC), Citrobacter rodentium (C. rodentium), and Cryptosporidium parvum (C. parvum). Potential therapeutic molecules were tested to detect effects on transepithelial resistance (TER), bacterial translocation (BT), claudin-4 expression, and regulation of the inflammatory cytokine response.
Results
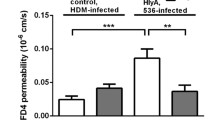
All three enteropathogens compared to uninfected cells, reduced TER (EPEC; p < 0.0001, C. rodentium; p < 0.0001, C. parvum; p < 0.0007), reduced claudin-4 expression, and permitted BT (EPEC; p < 0.0001, C. rodentium; p < 0.0001, C. parvum; p < 0.0003) through the monolayer. Zinc, colostrum, epidermal growth factor, trefoil factor 3, resistin-like molecule-β, hydrocortisone, and the myosin light chain kinase inhibitor ML7 (Hexahydro-1-[(5-iodo-1-naphthalenyl)sulfonyl]-1H-1,4-diazepine hydrochloride); ML7) improved TER (up to 70%) and decreased BT (as much as 96%). Only zinc demonstrated modest antimicrobial activity.
Conclusion
The enteropathogens impaired intestinal–epithelial barrier integrity with dysregulation of claudin-4 and increased bacterial translocation. Enteropathogen-mediated damage was reduced using contra-pathogenicity agents which mitigated the effects of pathogens without direct antimicrobial activity.








Similar content being viewed by others
References
Turner JR. Intestinal mucosal barrier function in health and disease. Ann Rev Immunol. 2009;9:799–809.
Salazar-Lindo E, Allen S, Brewster DR, Elliott EJ, et al. Intestinal infections and environmental enteropathy: working group report of the second world congress of pediatric gastroenterology, hepatology, and nutrition. J Pediatr Gastroenterol Nutr. 2004;39:S662–S669.
Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, et al. MAL-ED network. Investigators pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health. 2015;3:e564–e575.
Lee GO, McCormick BJJ, Seidman JC, Kosek MN, et al. For the MAL-ED network investigators. Infant nutritional status, feeding practices, enteropathogen exposure, socioeconomic status, and illness are associated with gut barrier function as assessed by the lactulose mannitol test in the MAL-ED birth cohort. Am J Trop Med Hyg. 2017;97:281–290.
Prendergast A, Kelly P. Enteropathies in the developing world; neglected effects on global health. Am J Trop Med Hyg. 2012;86:756–763.
Naylor C, Lu M, Haque R, Mondal D, et al. Environmental Enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine. 2015;2:1759–1766.
Manary MJ, Abrams SA, Griffin IJ, Quimper MM, et al. Perturbed zinc homeostasis in rural 3-5-y-old Malawian children is associated with abnormalities in intestinal permeability attributed to tropical enteropathy. Pediatr Res. 2010;67:671–675.
Tostman A, Mtabho CM, Semvua HH, van den Boogaard J, et al. Pharmacokinetics of first-line tuberculosis drugs in Tanzanian patients. Antimicrob Agents Chemother. 2013;57:3208–3213.
Campbell DI, Elia M, Lunn PG. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr. 2003;133:1332–1338.
Kelly P, Besa E, Zyambo K, Louis-Auguste J, et al. Endomicroscopic and transcriptomic analysis of impaired barrier function and malabsorption in environmental enteropathy. PLoS Negl Trop Dis. 2016;10:e0004600.
Korpe PS, Petri WA. Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med. 2012;18:328–336.
Prendergast AJ, Kelly P. Interactions between intestinal pathogens, enteropathy and malnutrition in developing countries. Curr Opin Infect Dis. 2016;29:229–236.
Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222.
Liu J, Platts-Mills JA, Juma J, Kabir F, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet. 2016;388:1291–1301.
Lima AAM, Leite ÁM, Di Moura A, Lima NL, et al. Determinant variables, enteric pathogen burden, gut function and immune-related inflammatory biomarkers associated with childhood malnutrition: a prospective case-control study in Northeastern Brazil. Pediatr Infect Dis J. 2017;36:1177–1185.
Amour C, Gratz J, Mduma E, Svensen E, et al. Etiology, Risk factors, and interactions of enteric infections and malnutrition and the consequences for child health and development project (MAL-ED) network investigators. Epidemiology and impact of campylobacter infection in children in 8 low-resource settings: results from the MAL-ED study. Clin Infect Dis. 2016;63:1171–1179.
Kosek MN, the MAL-ED Network Investigators. Causal pathways from enteropathogens to environmental enteropathy: findings from the MAL-ED birth cohort study. e-Biomedicine 2017; in press.
Marchiando AM, Shen L, Graham WV, Edelblum KL, et al. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology. 2011;140:1208–1218.
Flynn AN, Buret AG. Caspases-3, -8, and -9 are required for induction of epithelial cell apoptosis by enteropathogenic E. coli but are dispensable for increased paracellular permeability. Microb Pathog. 2008 Apr;44:311-9. Epub. 10/24/2007.
Tapia R, Kralicek SE, Hecht GA. Modulation of epithelial cell polarity by bacterial pathogens. Ann N Y Acad Sci. 2017;1405:16–24.
Shifflett DE, Clayburgh DR, Koutsouris A, Turner JR, Hecht GA. Enteropathogenic E. coli disrupts tight junction barrier function and structure in vivo. Lab Invest. 2005;85:1308–1324.
Kelly P, Menzies I, Crane R, Zulu I, et al. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. Am J Trop Med Hyg. 2004;70:412–419.
Amadi B, Mwiya M, Musuku J, Watuka A, et al. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet. 2002;360:1375–1380.
Deng W, Li Y, Vallance BA, Finlay BB. Locus of enterocyte effacement from Citrobacter rodentium: sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens. Infect Immun.. 2001;69:6323–6335.
Lai Y, Rosenshine I, Leong JM, Frankel G. Intimate host attachment: enteropathogenic and enterohaemorrhagic Escherichia coli. Cell Microbiol. 2013;15:1796–1808.
Yang J, Tauschek M, Hart E, Hartland EL, Robins-Browne RM. Virulence regulation in Citrobacter rodentium: the art of timing. Microb Biotechnol. 2010;3:259–268.
Liu J, Bolick DT, Kolling GL, Fu Z, Guerrant RL. Protein malnutrition impairs intestinal epithelial cell turnover, a potential mechanism of increased cryptosporidiosis in a murine model. Infect Immun. 2016;84:3542–3549.
Butler M, Ng CY, van Heel DA, Lombardi G, et al. Modulation of dendritic cell phenotype and function in an in vitro model of the intestinal epithelium. Eur J Immunol. 2006;36:864–874.
Moura RA, Sircili MP, Leomil L, Matté MH, et al. Clonal relationship among atypical enteropathogenic Escherichia coli strains isolated from different animal species and humans. Appl Environ Microbiol. 2009;75:7399–7408.
Moon HW, Whipp SC, Argenzio RA, Levine MM, Giannella RA. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–1351.
Amadi B, Besa E, Zyambo K, Kaonga P, et al. Impaired barrier function and autoantibody generation in malnutrition enteropathy in Zambia. EBioMedicine. 2017;22:191–199.
Flynn AN, Buret AG. Tight junctional disruption and apoptosis in an in vitro model of Citrobacter rodentium infection. Microb Pathog. 2008;45:98–104.
de Sablet T, Potiron L, Marquis M, Bussière FI, Lacroix-Lamandé S, Laurent F. Cryptosporidium parvum increases intestinal permeability through interaction with epithelial cells and IL-1β and TNFα released by inflammatory monocytes. Cell Microbiol. 2016;18:1871–1880.
De Rycke J, Comtet E, Chalareng C, Boury M, Tasca C, Milon A. Escherichia coli O103 from rabbit elicits actin stress fibers and focal adhesions in HeLa epithelial cells, cytopathic effects that are linked to an analog of the locus of enterocyte effacement. Infect Immun. 1997;65:2555–2563.
Higgins LM, Frankel G, Douce G, Dougan G, MacDonald TT. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect Immun. 1999;67:3031–3039.
Kumar A, Chatterjee I, Anbazhagan AN, Jayawardena D, et al. Cryptosporidium parvum disrupts intestinal epithelial barrier function via altering expression of key tight junction and adherens junction proteins. Cell Microbiol. 2018;20:e12830.
McNamara BP, Koutsouris A, O’Connell CB, Nougayréde JP, Donnenberg MS, Hecht G. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J Clin Invest. 2001;107:621–629.
Glotfelty LG, Zahs A, Hodges K, Shan K, Alto NM, Hecht GA. Enteropathogenic E. coli effectors EspG1/G2 disrupt microtubules, contribute to tight junction perturbation and inhibit restoration. Cell Microbiol. 2014;16:1767–1783.
Imdad A, Yakoob MY, Sudfeld C, Haider BA, Black RE, Bhutta ZA. Impact of vitamin A supplementation on infant and childhood mortality. BMC Public Health. 2011;11:S20.
Lazzerini M, Wanzira H. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev. 2016;2016:CD005436.
Turner JR, Buschmann MM, Sailer A, Calvo IR, Shen L. The role of molecular remodeling in differential regulation of tight junction permeability. Semin Cell Dev Biol. 2014;36:204–212.
Marie CS, Verkerke HP, Paul SN, Mackey AJ, Petri WA Jr. Leptin protects host cells from entamoeba histolytica cytotoxicity by a STAT3-dependent mechanism. Infect Immun. 2012;80:1934–1943.
Reeves PG, Briske-Anderson M, Johnson L. Pre-treatment of Caco-2 cells with zinc during the differentiation phase alters the kinetics of zinc uptake and transport (2). J Nutr Biochem. 2001;12:674–684.
Metzler-Zebeli BU, Caine WR, McFall M, Miller B, et al. Supplementation of diets for lactating sows with zinc amino acid complex and gastric nutriment-intubation of suckling pigs with zinc methionine on mineral status, intestinal morphology and bacterial translocation in lipopolysaccharide-challenged weaned pigs. J Anim Physiol Anim Nutr (Berl). 2010;94:237–249.
Hogan SP, Seidu L, Blanchard C, Groschwitz K, et al. Resistin-like molecule beta regulates innate colonic function: barrier integrity and inflammation susceptibility. J Allergy Clin Immunol. 2006;118:257–268.
Herbert DR, Yang JQ, Hogan SP, Groschwitz K, et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med. 2009;206:2947–2957.
Bergstrom KS, Morampudi V, Chan JM, Bhinder G, et al. Goblet cell derived RELM-β recruits CD4 + T cells during infectious colitis to promote protective intestinal epithelial cell proliferation. PLoS Pathog. 2015;11:e1005108.
Ting F, Znalesniak EB, Kalinski T, et al. TFF peptides play a role in the immune response following oral infection of mice with Toxoplasma gondii. Eur J Microbiol Immunol (Bp). 2015;5:221–231.
Sansonetti PJ, Arondel J, Huerre M, Harada A, Matsusima K. Interleukin-8 controls bacterial transepithelial translocation at the cost of epithelial destruction in experimental Shigellosis. Infect Immun. 1999;67:1471–1480.
Playford RJ, Macdonald CE, Johnson WS. Colostrum and milk-derived peptide growth factors for the treatment of gastrointestinal disorders. Am J Clin Nutr. 2000;72:5–14.
Playford RJ, Hanby AM, Gschmeissner S, Peiffer LP, et al. The epidermal growth factor receptor (EGF-R) is present on the basolateral, but not the apical, surface of enterocytes in the human gastrointestinal tract. Gut. 1996;39:262–266.
Okuyama H, Urao M, Lee D, Drongowski RA, Coran AG. The effect of epidermal growth factor on bacterial translocation in newborn rabbits. J Pediatr Surg. 1998;33:225–228.
Lu L, Li T, Williams G, Petit E, Borowsky M, Walker WA. Hydrocortisone induces changes in gene expression and differentiation in immature human enterocytes. Am J Physiol Gastrointest Liver Physiol. 2011;300:G425–G432.
Tian JQ, Göke M, Podolsky DK. Glucocorticoids have pleiotropic effects on small intestinal crypt cells. Am J Physiol. 1999;277:G1027–G1040.
Friedman J. The long road to leptin. J Clin Invest. 2016;126:4727–4734.
Cunningham KE, Turner JR. Myosin light chain kinase: pulling the strings of epithelial tight junction function. Ann N Y Acad Sci. 2012;1258:34–42.
Barrera GJ, Tortolero GS. Trefoil factor 3 (TFF3) from human breast milk activates PAR-2 receptors, of the intestinal epithelial cells HT-29, regulating cytokines and defensins. Bratisl Lek Listy. Infect Immun. 2008;76:796–811.
Kozakova H, Hanson LA, Stepankova R, Kahu H, Dahlgren UI, Wiedermann U. Vitamin A deficiency leads to severe functional disturbance of the intestinal epithelium enzymes associated with diarrhoea and increased bacterial translocation in gnotobiotic rats. Microbes Infect. 2003;5:405–411.
Wang X, Valenzano MC, Mercado JM, Zurbach EP, Mullin JM. Zinc supplementation modifies tight junctions and alters barrier function of CACO-2 human intestinal epithelial layers. Dig Dis Sci. 2013;58:77–87.
Acknowledgments
We gratefully acknowledge funding from Barts and The London Charity, and David Wareham (Blizard Institute, Barts and The London School of Medicine, UK) for a gift of E. coli K12.
Funding
Funding for this study was from Bart’s Charity and provided support in the form of salaries for authors [NC] but did not have any additional role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript. ME is an employee of Takeda Pharmaceuticals—This funder provided support in the form of salaries for authors [ME] but did not have any additional role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript. GJS is currently in receipt of funding from Takeda Pharmaceuticals, BBSRC together with 31, Benevolent and the Dunhill Foundation. He acts as a scientific advisor to 3 Takeda Pharmaceuticals and Zealand Pharma. “These funders provided support in the form of salaries for authors [GJS] but did not have any additional role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there are no conflicting interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Choudhry, N., Scott, F., Edgar, M. et al. Reversal of Pathogen-Induced Barrier Defects in Intestinal Epithelial Cells by Contra-pathogenicity Agents. Dig Dis Sci 66, 88–104 (2021). https://doi.org/10.1007/s10620-020-06121-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06121-9