Abstract
Objective
To assess the true efficacy of direct acting antiviral (DAA) therapy in real-world clinical practice, taking into account those patients that do not complete therapy or the necessary follow-up to establish sustained viral response (SVR).
Methods
Retrospective data collection of 261 genotype 1 HCV-infected patients, treatment naïve or treatment experienced, treated with ledipasvir/sofosbuvir combination therapy at an academic medical center. All patients received individualized teaching and counseling prior to starting therapy stressing importance of compliance with laboratory monitoring and treatment completion. Intention to treat SVR rates (ITT-SVR) and per-protocol SVR rates (PP-SVR) were calculated. Chi-squared test was used to compare the number of subjects lost to follow-up in the treatment-naïve vs. treatment-experienced groups. Characteristics of noncompliant patients were compared to compliant patients.
Results
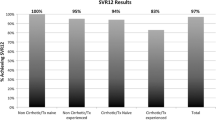
ITT-SVR rates for the entire cohort were 74%, significantly lower than the 95% PP-SVR rate for the compliant patients (p < 0.001). ITT-SVR was lower in treatment-naïve patients compared to treatment-experienced patients (68% vs. 86%). Among the entire cohort, 22% of patients either discontinued therapy prematurely (7%) or did not return for SVR assessment (15%). Failure to complete therapy or return for SVR assessment was statistically more common among treatment-naïve patients compared to treatment-experienced patients (28% vs. 11%, p = 0.0016).
Conclusions
There is a significant rate of noncompliance among patients treated with DAA in real-world clinical practice despite pre-treatment education efforts. The ITT-SVR rates observed in clinical practice were significantly lower than those reported by clinical trials, and this difference was most pronounced among treatment-naïve patients.


Similar content being viewed by others
References
Hagan LM, Schinazi RF. Best strategies for global HCV eradication. Liver Int. 2013;33:68–79.
Combating Hepatitis B and C to Reach Elimination by 2030. World Health Organization. www.who.int/hepatitis/publications/hep-elimination-by-2030-brief/en/; 2016. Accessed 13 Mar 2018.
Innes H, McAuely A, Alavi M, et al. The contribution of health risk behaviors to excess mortality in American adults with chronic hepatitis C: a population cohort study. Hepatology. 2018;67:97–107.
Singal AG, Volk ML, Jensen D, et al. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8:280–288.
Centers for Disease Control and Prevention, Division of Viral Hepatitis. Hepatitis C FAQs for health professionals. http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm. Accessed March 1, 2018.
Terrault NA, Zeuzem S, Di Bisceglie AM, et al. Effectiveness of Ledipasvir-Sofosbuvir combination in patients with hepatitis C virus infection and factors associated with sustained virologic response. Gastroenterology. 2016;151:1131–1140.
Kowdley KV, Sundaram V, Jeon CY, et al. Eight weeks of ledipasvir/sofosbuvir is effective for selected patients with genotype 1 hepatitis C infection. Hepatology. 2017;65:1094–1103.
Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the veterans affairs national health care system. Gastroenterology. 2016;151:457–471.
Buggisch P, Vermehren J, Mauss S, et al. Real-world effectiveness of 8-week treatment with ledipasvir/sofosbuvir in chronic hepatitis C. J Hepatol. 2018;68:663–671.
Falade-Nwulia O, Sutcliffe C, Moon J, et al. High hepatitis C cure rates among black and nonblack human immunodeficiency virus-infected adults in an urban center. Hepatology. 2017;66:1402–1412.
Innes H, McDonald S, Hayes P, et al. Mortality in hepatitis C patients who achieve a sustained viral response compared to the general population. J Hepatol. 2017;66:19–27.
Author information
Authors and Affiliations
Contributions
Both authors contributed to the planning and conduct of the study, the collection and interpretation of the data, the drafting of the manuscript, and the review of the final manuscript. Both authors have reviewed and approved this final manuscript submitted for publication.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Jorge L. Herrera is a member of the speaker’s bureau of Gilead Sciences Inc., and Abbvie Inc. Dr. Mary Caitlin Marshall has no competing interests.
Rights and permissions
About this article
Cite this article
Marshall, M.C., Herrera, J.L. Lack of Patient Compliance in Real-World Practice Negatively Affects Sustained Viral Response Rates to Direct Acting Agent Therapy for Hepatitis C. Dig Dis Sci 63, 3228–3232 (2018). https://doi.org/10.1007/s10620-018-5247-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5247-5