Abstract
Background
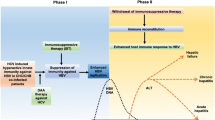
Practice guidelines recommend screening for hepatitis B virus (HBV) infection prior to initiating treatment of inflammatory bowel disease (IBD) with anti-tumor necrosis factor (anti-TNF) therapy. However, the adherence to these screening guidelines and the clinical outcomes of HBV reactivation following anti-TNF use are not well known.
Methods
This is a retrospective cohort study using the Veterans Health Administration datasets for IBD patients with filled prescriptions for anti-TNFs from 2003 to 2011. Laboratory testing was used to define HBV screening status in the 12 months preceding anti-TNF initiation. Logistic regression models were used to identify predictors of HBV screening. Cases of potential HBV reactivation were identified using ICD-9 codes for HBV infection or acute liver failure or by medications used for HBV infection treatment, and manually reviewed for verification.
Results
We identified 3357 IBD patients with filled prescriptions for anti-TNF medications. The HBV testing prior to anti-TNF initiation was 8.1% in 2003 and increased to 43.2% by 2011, with an overall rate of 23.7%. In multivariate analysis, African-American race, facilities with a higher volume of IBD patients, and facilities with an academic affiliation were associated with a higher probability of HBV screening. We did not identify a single case of confirmed clinically relevant HBV reactivation after anti-TNF initiation during 7210 patient-years of medication use.
Conclusions
HBV screening rates prior to anti-TNF initiation are low among IBD patients, but have increased over time. Despite low rates of screening, clinically significant HBV reactivation after anti-TNF initiation in this US cohort was nonexistent.

Similar content being viewed by others
References
Esteve M, Saro C, Gonzalez-Huix F, et al. Chronic hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: need for primary prophylaxis. Gut. 2004;53:1363–1365.
Ojiro K, Naganuma M, Ebinuma H, et al. Reactivation of hepatitis B in a patient with Crohn’s disease treated using infliximab. J Gastroenterol. 2008;43:397–401.
Esteve M, Loras C, Gonzalez-Huix F. Lamivudine resistance and exacerbation of hepatitis B in infliximab-treated Crohn’s disease patient. Inflamm Bowel Dis. 2007;13:1450–1451.
Morisco F, Castiglione F, Rispo A, et al. Effect of immunosuppressive therapy on patients with inflammatory bowel diseases and hepatitis B or C virus infection. J Viral Hepat. 2013;20:200–208.
Katsanos KH, Tsianos VE, Zois CD, et al. Inflammatory bowel disease and hepatitis B and C in Western Balkans: a referral centre study and review of the literature. J Crohns Colitis. 2010;4:450–465.
Papa A, Felice C, Marzo M, et al. Prevalence and natural history of hepatitis B and C infections in a large population of IBD patients treated with anti-tumor necrosis factor-alpha agents. J Crohns Colitis. 2013;7:113–119.
Chevaux JB, Nani A, Oussalah A, et al. Prevalence of hepatitis B and C and risk factors for nonvaccination in inflammatory bowel disease patients in Northeast France. Inflamm Bowel Dis. 2010;16:916–924.
Garcia-Vidal C, Rodriguez-Fernandez S, Teijon S, et al. Risk factors for opportunistic infections in infliximab-treated patients: the importance of screening in prevention. Eur J Clin Microbiol Infect Dis. 2009;28:331–337.
Loras C, Gisbert JP, Minguez M, et al. Liver dysfunction related to hepatitis B and C in patients with inflammatory bowel disease treated with immunosuppressive therapy. Gut. 2010;59:1340–1346.
Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–468.
van der Have M, Belderbos TD, Fidder HH, et al. Screening prior to biological therapy in Crohn’s disease: adherence to guidelines and prevalence of infections. Results from a multicentre retrospective study. Dig Liver Dis. 2014;46:881–886.
Gupta A, Macrae FA, Gibson PR. Vaccination and screening for infections in patients with inflammatory bowel disease: a survey of Australian gastroenterologists. Intern Med J. 2011;41:462–467.
Vaughn BP, Doherty GA, Gautam S, et al. Screening for tuberculosis and hepatitis B prior to the initiation of anti-tumor necrosis therapy. Inflamm Bowel Dis. 2012;18:1057–1063.
Hou JK, Tan M, Stidham RW, et al. Accuracy of diagnostic codes for identifying patients with ulcerative colitis and Crohn’s disease in the Veterans Affairs Health Care System. Dig Dis Sci. 2014;59:2406–2410.
Hwang JP, Fisch MJ, Zhang H, et al. Low rates of hepatitis B virus screening at the onset of chemotherapy. J Oncol Pract. 2012;8:e32–e39.
Stine JG, Bass M, Ibrahim D, et al. Dermatologists’ awareness of and screening practices for hepatitis B virus infection before initiating tumor necrosis factor-alpha inhibitor therapy. South Med J. 2011;104:781–788.
Stine JG, Khokhar OS, Charalambopoulos J, et al. Rheumatologists’ awareness of and screening practices for hepatitis B virus infection prior to initiating immunomodulatory therapy. Arthritis Care Res. 2010;62:704–711.
Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:e3.
Noska AJ, Belperio PS, Loomis TP, et al. Prevalence of Human Immunodeficiency Virus, Hepatitis C Virus, and Hepatitis B Virus among homeless and nonhomeless United States Veterans. Clin Infect Dis. 2017;65:252–258.
Weinbaum CM, Williams I, Mast EE, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1–20.
Thirumurthi S, Chowdhury R, Richardson P, et al. Validation of ICD-9-CM diagnostic codes for inflammatory bowel disease among veterans. Dig Dis Sci. 2010;55:2592–2598.
Herrinton LJ, Liu L, Lafata JE, et al. Estimation of the period prevalence of inflammatory bowel disease among nine health plans using computerized diagnoses and outpatient pharmacy dispensings. Inflamm Bowel Dis. 2007;13:451–461.
Acknowledgments
JH has served as a speaker for Abbvie, Janssen, a consultant for UCB, and served on an advisory board for Pfizer. JH has received research funding from Abbvie, Janssen, Pfizer, Celgene, and Redhill Biopharma. EH, RS, JK, PR, SS, and HE have no financial disclosures.
Funding
The research reported here was supported in part by the American College of Gastroenterology Junior Faculty Development Award (JKH) and with resources at the VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (#CIN 13-413), at the Michael E. DeBakey VA Medical Center, Houston, TX (JKH). No writing assistance was used in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
JH contributed to study design, data analysis, and authorship of manuscript. He has approved the final draft submitted. EYH contributed to authorship and editorial input of the manuscript. She has approved the final draft submitted. JRK contributed to study design, data analysis, and editorial input in the manuscript. She has approved the final draft submitted. PR contributed to study design, programming, data abstraction, data analysis, and editorial input in the manuscript. He has approved the final draft submitted. SS contributed to study design, programming, data abstraction, data analysis, and editorial input in the manuscript. She has approved the final draft submitted. HE contributed to study design, data interpretation, and editorial input in the manuscript. He has approved the final draft submitted. RS contributed to authorship and editorial input of the manuscript. He has approved the final draft submitted.
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, R., Ho, E.Y., Kramer, J.R. et al. Hepatitis B Virus Screening and Reactivation in a National VA Cohort of Patients with Inflammatory Bowel Disease Treated with Tumor Necrosis Factor Antagonists. Dig Dis Sci 63, 1551–1557 (2018). https://doi.org/10.1007/s10620-018-5042-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5042-3