Abstract
Background
Intestinal mucosal barrier dysfunction can be caused by severe acute pancreatitis (SAP). It is normally associated with changes to mucosal autophagy and oxidative stress.
Objective
The aim of this study was to investigate the correlation between autophagy and oxidative stress on the intestinal mucosal barrier of SAP rat model.
Methods
SAP was induced by retrograde injection of sodium taurocholate (5%) into the biliopancreatic duct. Bacterial translocation (BT) was detected by 16S rDNA sequencing analysis. Morphological alterations in the pancreas and gut were determined by hematoxylin–eosin staining. Oxidative stress status was determined by measuring the level of intestinal malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione peroxidase (GPx). Western blot, RT-PCR, and immunofluorescent staining were preformed to analyze the expression of tight junction and autophagy proteins.
Results
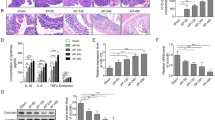
According to the sequencing analysis, rats in SAP group were divided into BT (+) group (n = 9) and BT (−) group (n = 8). Pancreatic and intestinal injuries in SAP group were significantly higher than sham operation group. The content of MDA was clearly elevated, and SOD as well as GPx activities were decreased in BT (+) group as compared with BT (−) group. The expression of LC3II and Beclin1 in BT (−) group was higher than that observed in BT (+). In contrast, BT (+) group had a higher level of claudin-2 and a lower level of zonula occluden-1, occludin, and claudin-1.
Conclusion
These results suggest that activated autophagy may attenuate intestinal mucosal barrier dysfunction by preventing and reducing the oxidative stress in SAP.







Similar content being viewed by others
References
Singh VK, Wu BU, Bollen TL, et al. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clin Gastroenterol Hepatol. 2009;7:1247–1251.
Pinzone MR, Celesia BM. Microbial translocation in chronic liver diseases. Int J Microbiol. 2012;2012:694629.
Lee J, Mo JH, Katakura K, et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signaling in intestinal epithelial cells. Nat Cell Biol. 2007;8:1327–1336.
Jiang Y, Lin J, Zhang D, et al. Bacterial translocation contributes to cachexia and its possible pathway in patients with colon cancer. J Clin Gastroenterol. 2014;48:131.
Wang J, Li C, Jiang Y, et al. Effect of ceramide-1-phosphate transfer protein on intestinal bacterial translocation in severe acute pancreatitis. Clin Res Hepatol Gastroenterol. 2016;41:86.
Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119.
Umeda K, Ikenouchi J, Katahira-Tayama S, et al. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754.
Jiang Y, Guo C, Zhang D, Zhang J, Wang X, Geng C. The altered tight junctions: an important gateway of bacterial translocation in cachexia patients with advanced gastric cancer. J Interferon Cytokine Res. 2014;34:518.
Huang FC. De Novo sphingolipid synthesis is essential for Salmonella-induced autophagy and human beta-defensin 2 expression in intestinal epithelial cells. Gut Pathog. 2016;8:1–11.
Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861.
Inoue J, Nishiumi S, Fujishima Y, et al. Autophagy in the intestinal epithelium regulates Citrobacter rodentium infection. Arch Biochem Biophys. 2012;521:95–101.
Randall-Demllo S, Chieppa M, Eri R. Intestinal epithelium and autophagy: partners in gut homeostasis. Front Immunol. 2013;4:301.
Nighot PK, Hu CA, Ma TY. Autophagy enhances intestinal epithelial tight junction barrier function by targeting claudin-2 protein degradation. J Biol Chem. 2015;290:7234.
Saito S, Nakashima A. A review of the mechanism for poor placentation in early-onset preeclampsia: the role of autophagy in trophoblast invasion and vascular remodeling. J Reprod Immunol. 2014;101–102:80.
Huang J, Lam GY, Brumell JH. Autophagy signaling through reactive oxygen. species. Antioxid Redox Signal. 2011;14:2215–2231.
Wu YT, Tan HL, Huang Q, et al. Autophagy plays a protective role during zVAD-induced necrotic cell death. Autophagy. 2008;4:457.
Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013;13:722–737.
Wen W, Zheng H, Jiang Y, et al. Effect of intestinal epithelial autophagy on bacterial translocation in severe acute pancreatitis. Clin Res Hepatol Gastroenterol. 2017;41:703–710.
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739.
Schmidt J, Rattner DW, Lewandrowski K, et al. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44.
Chiu CJ, Mcardle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478.
Ren W, Liu S, Chen S, et al. Dietary l-glutamine supplementation increases Pasteurella multocida burden and the expression of its major virulence factors in mice. Amino Acids. 2013;45:947.
Amasheh S, Dullat S, Fromm M, Schulzke JD, Buhr HJ, Kroesen AJ. Inflamed pouch mucosa possesses altered tight junctions indicating recurrence of inflammatory bowel disease. Int J Colorectal Dis. 2009;24:1149–1156.
Liang HY, Chen T, Wang T, Huang Z, Yan HT, Tang LJ. Time course of intestinal barrier function injury in a sodium taurocholate-induced severe acute pancreatitis in rat model. J Dig Dis. 2014;15:386–393.
Cacopardo B, Pinzone, Berretta S, et al. Localized and systemic bacterial infections in necrotizing pancreatitis submitted to surgical necrosectomy or percutaneous drainage of necrotic secretions. BMC Surg. 2013;13:1–4.
Gosiewski T, Flis A, Sroka A, et al. Comparison of nested, multiplex, qPCR; FISH; SeptiFast and blood culture methods in detection and identification of bacteria and fungi in blood of patients with sepsis. BMC Microbiol. 2014;14:313.
Wu LL, Chiu HD, Peng WH, et al. Epithelial inducible nitric oxide synthase causes bacterial translocation by impairment of enterocytic tight junctions via intracellular signals of Rho-associated kinase and protein kinase C zeta. Crit Care Med. 2011;39:2087–2098.
Meriläinen S, Mäkelä J, Koivukangas V, et al. Intestinal bacterial translocation and tight junction structure in acute porcine pancreatitis. Hepatogastroenterology. 2012;59:599.
Deng WS, Zhang J, Lu H, et al. Arpin contributes to bacterial translocation and development of severe acute pancreatitis. World J Gastroenterol. 2015;21:4293.
Zhou H, Gao J, Wu W, et al. Octreotide ameliorates intestinal dysmotility by interstitial cells of Cajal protection in a rat acute necrotizing pancreatitis model. Pancreas. 2011;40:1226.
Baumgart DC, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care. 2002;5:685.
Kurihara Y, Kanki T, Aoki Y, et al. Mitophagy plays an essential role in reducing mitochondrial production of reactive oxygen species and mutation of mitochondrial DNA by maintaining mitochondrial quantity and quality in yeast. J Biol Chem. 2012;287:3265–3272.
Higgins GC, Coughlan MT. Mitochondrial dysfunction and mitophagy: the beginning and end to diabetic nephropathy? Br J Pharmacol. 2014;171:1917–1942.
Tang Y, Li J, Li F, et al. Autophagy protects intestinal epithelial cells against deoxynivalenol toxicity by alleviating oxidative stress via IKK signaling pathway. Free Radic Biol Med. 2015;89:944.
Zhu H, Foretz M, Xie Z, et al. PRKAA1/AMPKα1 is required for autophagy-dependent mitochondrial clearance during erythrocyte maturation. Autophagy. 2014;10:1522–1534.
Zhang H, Kong X, Kang J, et al. Oxidative stress induces parallel autophagy and mitochondria dysfunction in human glioma U251 cells. Toxicol Sci. 2009;110:376.
Acknowledgments
This work was supported by the National Natural Science Foundation of China, Nos. 81270448 and 81470890.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Huang, L., Jiang, Y., Sun, Z. et al. Autophagy Strengthens Intestinal Mucosal Barrier by Attenuating Oxidative Stress in Severe Acute Pancreatitis. Dig Dis Sci 63, 910–919 (2018). https://doi.org/10.1007/s10620-018-4962-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-4962-2