Abstract
Background
Previous studies suggested that cytochrome P450 participated in the tumor metastasis and migration. CYP2B6 also acts as an important enzyme which metabolize partially or primarily metabolism of drugs, environmental contaminants, and mutagens. The objective of this study was to investigate the influence of CYP2B6 polymorphism on susceptibility of Hirschsprung disease.
Methods
TaqMan assay was performed to determine the genotypes of CYP2B6 rs707265, rs1042389, rs2054675 in 262 cases and 290 control subjects. Logistic regression was used to assess the associations between these polymorphisms and HSCR.
Results
We observed a significant association of CYP2B6 rs707265 (G>A) polymorphism and HSCR susceptibility (p < 0.001). Besides, rs707265 A presented a significant risk of HSCR (p < 0.001).
Conclusions
Our result suggested that CYP2B6 rs707265 modified the risk of HSCR.
Similar content being viewed by others
Introduction
Hirschsprung’s disease (HSCR) is a rare disease characterized by the absence of ganglion cells along a variable length in the gastrointestinal tract, which results in the contraction of the aganglionic gut segment and functional intestinal obstruction [1]. HSCR is mainly caused by a failure in migration of enteric neural crest cells into the intestinal tract, or due to a failure in survival, proliferation, or differentiation of enteric neural crest cells once they reach the gut. It has a morbidity of 1/5,000 live births and is most prevalent among Asians [2]. Moreover, males are affected about 3.5–7.8 times more than females [3]. According to the extent of aganglionosis, HSCR patients can be classified as short-segment HSCR (S-HSCR, 80 % of cases), when the aganglionic segment does not extend beyond the upper sigmoid, and long segment HSCR (L-HSCR, 20 % of cases), when aganglionosis extends proximal to the sigmoid [4]. Clinical manifestations of HSCR include constipation, distension of the proximal colon, and concomitant complications [5].
CYP2B6, which is located on chromosome 19, codes metabolic enzyme of the Cytochrome P450 family [6, 7]. CYP2B6 acts as the phase I metabolic enzyme and plays a key role in the biotransformation of many xenobiotics [8]. If the xenobiotics as precarcinogens transform to their biologically active forms, they then may irreversibly react with DNA to cause mutations, chromosomal aberrations, and cancer, including congenital disorder such as HSCR [9]. The human gene coding for CYP2B6 is highly polymorphic while the hepatic content and activity of the enzyme itself are subject to wide variation [10]. It was reported that CYP2B6 polymorphism was associated with some diseases, such as leukemia [11]. In this study, we proposed to understand whether genetic variants in CYP2B6 were associated with HSCR.
Methods
Patients and Blood Samples
The Institutional Ethics Committee approval for the project was obtained before the study started. Activities involving subjects were performed in accordance with the declaration of Helsinki. A total of 262 blood samples were recruited from HSCR patient acquired surgical treatment in Nanjing Children’s Hospital affiliated to Nanjing Medical University and Xuzhou Children’s Hospital Affiliated to Xuzhou medical college from July 2010 to 2013. Pathological detection was performed after surgery in order to verify that the ganglion cells could not be detected in the aganglionic segments. A total of 290 samples in control group were blood obtained from patients undergoing surgical treatment for intussusception or incarcerated and strangulated inguinal hernia, and all samples were proved without HSCR or other congenital malformation. Patients and controls were matched by ethnicity, mean age, and gender. Both the HSCR and control group samples were collected after obtaining informed consent from their guardians for the collection of the blood. The blood tissues were immediately frozen and stored at −20 °C.
Genomic DNA Extraction and Polymorphism Genotyping
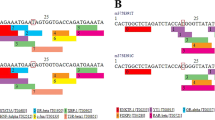
Blood samples were obtained from each individual and used for DNA extraction. Genomic DNA was extracted by using a DNA extraction kit (Tiangen Biotech, Beijing, China) according to the manufacturer’s instructions. SNPs of the CYP2B6 gene (rs707265, rs1042389, and rs2054675) were genotyped by the TaqMan Probe method on the ABI 7900 Real-time PCR system (Applied Biosystems, Foster City, CA, USA). SDS 2.4 software was used for allelic discrimination. For quality control, four negative controls were included in each plate, and 5 % of the samples were randomly selected for repeated genotyping to verify the results; all of the results were 100 % consistent. These SNPs (rs707265, rs1042389, and rs2054675) had been reported to be associated with other diseases or located in potential function areas, and the minor allele frequencies (MAF) value is greater than 0.05 in Chinese population.
Statistical Analysis
Genotype frequencies of all polymorphisms were tested for Hardy–Weinberg equilibrium. Statistical analysis was performed using Stata software (version 7.0, Stata Corp LP, College Station, TX, USA). Demographic characteristics of patients and controls were described as frequencies and percentages. Statistical significance of frequency differences between patients and control groups was evaluated using the Chi-squared test as well as the disease classification and genotype. Statistical odds ratios (OR) for risk prediction were derived by logistic regression analysis, and estimation was made with 95 % confidence intervals (95 % CI). Bonferroni adjustment was used to control the error rate due to multiple testing. As the result, a p value lower than 0.0167 (0.05/3) was considered statistically significant.
Results
The genotype distribution among controls and patients was in Hardy–Weinberg equilibrium. Distributions of the alleles and genotypes for SNPs were presented in Table 1. Significant associations were observed between the allele frequencies of rs707265 and the risk of HSCR (p < 0.001) (Table 2). The frequency of the homozygous AA genotype of rs707265 was lower in control group (Table 1). We observed higher frequency of heterozygous GA genotype of rs707265 in case group (p = 0.001, adjusted OR 2.09, 95 % CI 1.36––3.19 in GA genotype group) (Table 1), and the difference between combined variant genotypes GA\AA and GG was also significant (p < 0.001, adjusted OR 2.98, 95 % CI 2.00–4.45).
There were no significant differences between allele frequencies of rs1042389 T and C in cases and controls (p = 0.663) (Table 2). Genotype frequencies were of no significant difference between cases and controls (Table 1). As for rs2054675 (T>C), the genotype frequencies were TT 67.56 %, TC 29.01 %, CC 3.43 % in cases and TT 64.83 %, TC 32.41 %, CC 2.76 % in controls (Table 1). The allele frequencies were T 82.06 %, C 17.94 % in cases and T 81.03 %, C 18.97 % in control subjects (Table 2). We found no difference in the rs1042389 and rs2054675 genotype frequencies or allele frequencies between cases and controls.
For the relationship between disease classification and genotype, no significant associations were observed (Table 3).
Discussion
A number of studies had implicated defects in neurons since the early 1900s, but it was not found that all postmortem samples of rectum from patients with congenital megacolon lacked enteric (intrinsic) neurons until the mid-1900s [12]. It has been assumed that all cases of HSCR have a genetic basis, but mutations in genes associated with HSCR account for only about 50 % of familial cases of HSCR and 15 % of sporadic cases of HSCR [2]. Experimental results have confirmed that vitamin A deficiency increases the penetrance and severity of aganglionosis in a mouse model of HSCR [13]. Thus, environmental factors could contribute to susceptibility to HSCR [14].
The metabolism of environmental factors mainly performed by metabolic enzymes; however, CYP2B6 is an important metabolic enzyme. CYP2B6 protein is mainly expressed in the liver, which contributes 2–5 % of the total liver CYP content and exhibits about 300-fold variability of expression [15], but can be also expressed in the kidneys, intestines, skin, brain, and lungs [16]. CYP2B6 has been found to metabolize partially or primarily metabolism of many important drugs, environmental contaminants, and mutagens [9]. In vitro studies have shown that overexpression of CYP (CYP2C9) elicits angiogenesis via activation of the epidermal growth factor receptor (EGFR) [17], which plays an important role in migration. The overexpression of CYP epoxygenase enhanced tumor metastasis of human breast carcinoma cells to the lungs of athymic BALB/C and also notably enhanced the migration, invasion in a variety of cancer cell lines in vitro [17, 18]. Moreover, enteric neural crest cells migration disorder could be important in the pathogenesis of HSCR. Thus, we hypothesize that CYP2B6 affected migration of enteric neural crest cells and modified the risk of HSCR.
Genetic variations such as single nucleotide polymorphisms (SNPs) were thought to be important in determining the biological basis of complex genetic conditions. There were several polymorphisms in RET, NRG1, SEMA3A genes, which have been known to be risk factors for HSCR [3, 19]. Our study is the first to suggest associations between CYP2B6 polymorphisms and HSCR risk. In this paper, a total of 552 individuals were analyzed. For the SNP (rs1042389 and rs2054675), we did not detect any significant difference in genotype distribution or allele frequency between cases and controls. The results indicated that rs1042389 and rs2054675 in CYP2B6 had no association with susceptibility or risk to HSCR in the Chinese Han population. We found that the CYP2B6 rs707265 AA genotypes were associated with a high risk on HSCR compared with the GG genotype. Compared with G allele, the A allele (allele frequency 60.50 % in cases, 30.40 % from dbSNP) may have a high susceptibility to HSCR (p < 0.001). Because of this, we suspect that rs707265 (G>A) polymorphism may result in different capabilities to dispose of exogenous contaminants and impact on the expression and function of CYP2B6 proteins, which influencing the risk of HSCR.
In conclusion, we found CYP2B6 rs706252 (G>A) was associated with HSCR in Chinese Han population. However, further studies are needed to better understand the potential mechanisms.
References
Lecerf L, Kavo A, Ruiz-Ferrer M, et al. An impairment of long distance SOX10 regulatory elements underlies isolated Hirschsprung disease. Hum Mutat. 2014;35:303–307.
Amiel J, Sproat-Emison E, Garcia-Barcelo M, et al. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet. 2008;45:1–14.
Phusantisampan T, Sangkhathat S, Phongdara A, Chiengkriwate P, Patrapinyokul S, Mahasirimongkol S. Association of genetic polymorphisms in the RET-protooncogene and NRG1 with Hirschsprung disease in Thai patients. J Hum Genet. 2012;57:286–293.
N-Fékété C, Ricour C, Martelli H, Jacob SL, Pellerin D. Total colonic aganglionosis (with or without ileal involvement): a review of 27 cases. J Pediatr Surg. 1986;21:251–254.
Liang CM, Ji DM, Yuan X, Ren LL, Shen J, Zhang HY. RET and PHOX2B genetic polymorphisms and Hirschsprung’s disease susceptibility: a meta-analysis. PLoS One. 2014;9:e90091.
Fonseca F, de la Torre R, Diaz L, et al. Contribution of cytochrome P450 and ABCB1 genetic variability on methadone pharmacokinetics, dose requirements, and response. PLoS One. 2011;6:e19527.
Li Y, Kantelip JP, Gerritsen-van Schieveen P, Davani S. Interindividual variability of methadone response: impact of genetic polymorphism. Mol Diagn Ther. 2008;12:109–124.
Hodgson E, Rose RL. The importance of cytochrome P450 2B6 in the human metabolism of environmental chemicals. Pharmacol Ther. 2007;113:420–428.
Code EL, Crespi CL, Penman BW, Gonzalez FJ, Chang TK, Waxman DJ. Human cytochrome P4502B6: interindividual hepatic expression, substrate specificity, and role in procarcinogen activation. Drug Metab Dispos Biol Fate Chem. 1997;25:985–993.
Court MH, Duan SX, Hesse LM, Venkatakrishnan K, Greenblatt DJ. Cytochrome P-450 2B6 is responsible for interindividual variability of propofol hydroxylation by human liver microsomes. Anesthesiology. 2001;94:110–119.
Yuan ZH, Liu Q, Zhang Y, Liu HX, Zhao J, Zhu P. CYP2B6 gene single nucleotide polymorphisms and leukemia susceptibility. Ann Hematol. 2011;90:293–299.
Whitehouse FR, Kernohan JW. Myenteric plexus in congenital megacolon; study of 11 cases. Arch Intern Med. 1948;82:75–111.
Fu M, Sato Y, Lyons-Warren A, et al. Vitamin A facilitates enteric nervous system precursor migration by reducing Pten accumulation. Development. 2010;137:631–640.
McKeown SJ, Stamp L, Hao MM, Young HM. Hirschsprung disease: a developmental disorder of the enteric nervous system. Wiley Interdiscip Rev Dev Biol. 2013;2:113–129.
Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103–141.
Miksys S, Lerman C, Shields PG, Mash DC, Tyndale RF. Smoking, alcoholism and genetic polymorphisms alter CYP2B6 levels in human brain. Neuropharmacology. 2003;45:122–132.
Michaelis UR, Fisslthaler B, Medhora M, Harder D, Fleming I, Busse R. Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor (EGFR). FASEB J Off Publ Fed Am Soc Exp Biol. 2003;17:770–772.
Jiang JG, Ning YG, Chen C, et al. Cytochrome p450 epoxygenase promotes human cancer metastasis. Cancer Res. 2007;67:6665–6674.
Wang LL, Zhang Y, Fan Y, et al. SEMA3A rs7804122 polymorphism is associated with Hirschsprung disease in the Northeastern region of China. Birth Defects Res A Clin Mol Teratol. 2012;94:91–95.
Acknowledgments
We thank Dr. Xiaoqun Xu, Weiwei Jiang, Xiaofeng Lv, and Changgui Lu (Nanjing Children’s Hospital Affiliated to Nanjing Medical University) for sample collection. Grant support: Natural Science Foundation of China, Grant Number: 81370473. Natural Science Foundation of Jiangsu Province of China, Grant Number: BK20131388. Scientific Research Project of Jiangsu Provincial Department of Health, Grant Number: H201342. New Century Excellent Talents of MOE, Grant Number: NCET-13-0870. Priority Academic Program Development of Jiangsu Higher Education Institutions SKLRM-KF-1104. Nanjing Science and Technique Development Foundation (201108010) and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Chao Xu, Pingfa Chen and Hua Xie have contributed equally to this work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Xu, C., Chen, P., Xie, H. et al. Associations Between CYP2B6 rs707265, rs1042389, rs2054675, and Hirschsprung Disease in a Chinese Population. Dig Dis Sci 60, 1232–1235 (2015). https://doi.org/10.1007/s10620-014-3450-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3450-6