Abstract
Background and Aim
Nonalcoholic fatty liver disease (NAFLD) is strongly associated with obesity, hyperlipidemia, and type 2 diabetes mellitus. Several studies have found that fat mass and the obesity-associated (FTO) gene is linked to obesity. The aim of this work is to investigate the expression and function of FTO in liver with lipid metabolism diseases.
Methods
We investigated the basal FTO expression in an NAFLD rat model and compared it with control subjects. The function of FTO in lipid metabolism was further studied in L02 cells through overexpression experiments.
Results
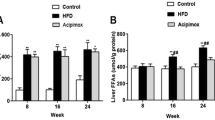
A significant increase in FTO mRNA and protein levels was found in the NAFLD group. In addition, the FTO levels were positively associated with malondialdehyde and superoxide dismutase concentrations. FTO overexpression in L02 cells enhanced lipogenesis and oxidative stress.
Conclusions
This study demonstrates that increased FTO levels in the liver are involved in oxidative stress and lipid deposition, which characterize NAFLD.





Similar content being viewed by others
References
Bellentani S, Marino M. Epidemiology and natural history of non-alcoholic fatty liver disease (NAFLD). Ann Hepatol. 2009;8(Suppl 1):S4–S8.
Targher G, Zoppini G, Day CP. Risk of all-cause and cardiovascular mortality in patients with chronic liver disease. Gut. 2011;60(11):1602–1603.; author reply 1603-4.
Scorletti E, Calder PC, Byrne CD. Non-alcoholic fatty liver disease and cardiovascular risk: metabolic aspects and novel treatments. Endocrine. 2011;40(3):332–343.
Thiruvagounder M, Khan S, Sheriff DS. Non-alcoholic fatty liver disease (NAFLD)—Is it an emerging risk factor for coronary artery disease? Preliminary study in a local Indian population. Sultan Qaboos Univ Med J. 2010;10(2):221–226.
Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol. 2009;104(4):861–867.
Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012;52(1):59–69.
Kuppan G, Anjana RM, Deepa M, et al. Inflammatory markers in relation to nonalcoholic fatty liver disease in urban South Indians. Diabetes Technol Ther. 2012;14(2):152–158.
Zhao L, Chen Y, Tang R, et al. Inflammatory stress exacerbates hepatic cholesterol accumulation via increasing cholesterol uptake and de novo synthesis. J Gastroenterol Hepatol. 2011;26(5):875–883.
Abdelmegeed MA, Yoo SH, Henderson LE, Gonzalez FJ, Woodcroft KJ, Song BJ. PPARalpha expression protects male mice from high fat-induced nonalcoholic fatty liver. J Nutr. 2011;141(4):603–610.
Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med. 2000;21(3):49–98.
Zhang BJ, Xu D, Guo Y, Ping J, Chen LB, Wang H. Protection by and anti-oxidant mechanism of berberine against rat liver fibrosis induced by multiple hepatotoxic factors. Clin Exp Pharmacol Physiol. 2008;35(3):303–309.
Zhu L, Fukuda S, Cordis G, Das DK, Maulik N. Anti-apoptotic protein survivin plays a significant role in tubular morphogenesis of human coronary arteriolar endothelial cells by hypoxic preconditioning. FEBS Lett. 2001;508(3):369–374.
Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–894.
McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med. 2010;363(24):2339–2350.
Kilpelainen TO, den Hoed M, Ong KK, et al. Obesity-susceptibility loci have a limited influence on birth weight: a meta-analysis of up to 28,219 individuals. Am J Clin Nutr. 2011;93(4):851–860.
Wahlen K, Sjolin E, Hoffstedt J. The common rs9939609 gene variant of the fat mass- and obesity-associated gene FTO is related to fat cell lipolysis. J Lipid Res. 2008;49(3):607–611.
Bravard A, Lefai E, Meugnier E, et al. FTO is increased in muscle during type 2 diabetes, and its overexpression in myotubes alters insulin signaling, enhances lipogenesis and ROS production, and induces mitochondrial dysfunction. Diabetes. 2011;60(1):258–268.
Doege H, Grimm D, Falcon A, et al. Silencing of hepatic fatty acid transporter protein 5 in vivo reverses diet-induced non-alcoholic fatty liver disease and improves hyperglycemia. J Biol Chem. 2008;283(32):22186–22192.
Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3(4):445–451.
Sozio MS, Liangpunsakul S, Crabb D. The role of lipid metabolism in the pathogenesis of alcoholic and nonalcoholic hepatic steatosis. Semin Liver Dis. 2010;30(4):378–390.
Fredriksson R, Hagglund M, Olszewski PK, et al. The obesity gene, FTO, is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology. 2008;149(5):2062–2071.
Olszewski PK, Fredriksson R, Olszewska AM, et al. Hypothalamic FTO is associated with the regulation of energy intake not feeding reward. BMC Neurosci. 2009;10:129.
Fischer J, Koch L, Emmerling C, et al. Inactivation of the FTO gene protects from obesity. Nature. 2009;458(7240):894–898.
Gerken T, Girard CA, Tung YC, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318(5855):1469–1472.
Acknowledgments
This work was supported by National Natural Science Foundation of China (30971405, 81170252, 81070656), the National Basic Research Program of China (2010CB535008, 973 Program), Medical Academic Key Talent Program of Jiangsu Province in China (2007200), the Jiangsu Province Innovation Project for Graduate Students of China (CXZZ11_0708), the China Postdoctoral Science Foundation (2012M510850) and Shanghai Postdoctoral Sustentation Fund (12R21414700).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jianjin Guo and Wei Ren contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, J., Ren, W., Li, A. et al. Fat Mass and Obesity-Associated Gene Enhances Oxidative Stress and Lipogenesis in Nonalcoholic Fatty Liver Disease. Dig Dis Sci 58, 1004–1009 (2013). https://doi.org/10.1007/s10620-012-2516-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2516-6