Abstract
Background
Abnormal expression of early growth response gene 1 (Egr-1) and β-catenin may play a crucial role in the development and progression of human cancer. However, little is known about the expression and underlying molecular mechanisms in which Egr-1 and β-catenin are involved in the development and progression of gastric cancer.
Aims
The purpose of this study was to elucidate the potential relationship between Egr-1 and β-catenin expression in gastric cancer, which contributes to finding new molecular carcinogenesis as a potential therapeutic target for gastric cancer.
Methods
In a sample of 102 cases of human gastric cancer, the expression of Egr-1 and β-catenin was detected using immunohistochemistry. Egr-1 gene was transfected into gastric cancer SGC7901 cells and its role in proliferation and cell invasion was detected by MTT assay, flow cytometry, wound-healing and transwell invasion assay. Western blot analysis was used to study the expression of β-catenin and cyclin D1 proteins.
Results
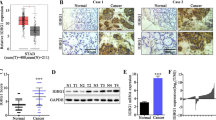
Upregulated Egr-1 and β-catenin protein expression were strongly correlated with cancer progression and depth of invasion in gastric cancer. β-catenin, present mainly in cytoplasmic and nucleus of gastric cancer cells, was also positively correlated with Egr-1 expression in gastric cancer. Furthermore, the overexpression of Egr-1 upregulated β-catenin expression level, promoted cell proliferation, increased cell population in S-phase and enhanced gastric cancer cell migration and invasion in vitro.
Conclusions
Egr-1 might contribute to gastric cancer proliferation and invasion through activation of the β-catenin signaling pathway.



Similar content being viewed by others
References
Baron V, Adamson ED, Calogero A, Ragona G, Mercola D. The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFbeta1, PTEN, p53, and fibronectin. Cancer Gene Ther. 2006;13:115–124.
Adamson E, de Belle I, Mittal S, et al. Egr1 signaling in prostate cancer. Cancer Biol Ther. 2003;2:617–622.
Abdulkadir SA. Mechanisms of prostate tumorigenesis: roles for transcription factors Nkx3.1 and Egr1. Ann N Y Acad Sci. 2005;1059:33–40.
Virolle T, Krones-Herzig A, Baron V, De Gregorio G, Adamson ED, Mercola D. Egr1 promotes growth and survival of prostate cancer cells. Identification of novel Egr1 target genes. J Biol Chem. 2003;278:11802–11810.
Sauer L, Gitenay D, Vo C, Baron VT. Mutant p53 initiates a feedback loop that involves Egr-1/EGF receptor/ERK in prostate cancer cells. Oncogene. 2010;29:2628–2637.
Boone DN, Qi Y, Li Z, Hann SR. Egr1 mediates p53-independent c-Myc induced apoptosis via a non canonical ARF-dependent transcriptional mechanism. Proc Natl Acad Sci USA. 2011;108:632–637.
Shareef MM, Cui N, Burikhanov R, Gupta S, Satishkumar S, et al. Role of tumor necrosis factor-alpha and TRAIL in high-dose radiation-induced bystander signaling in lung adenocarcinoma. Cancer Res. 2007;67:11811–11820.
Kobayashi D, Yamada M, Kamagata C, et al. Overexpression of early growth response-1 as a metastasis-regulatory factor in gastric cancer. Anticancer Res. 2002;22:3963–3970.
Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24.
Takasu S, Tsukamoto T, Cao XY, et al. Roles of cyclooxygenase-2 and microsomal prostaglandin E synthase-1 expression and beta-catenin activation in gastric carcinogenesis in N-methyl-N-nitrosourea-treated K19–C2mE transgenic mice. Cancer Sci. 2008;99:2356–2364.
Yoon CS, Hyung WJ, Lee JH, et al. Expression of S100A4, E-cadherin, alpha- and beta-catenin in gastric adenocarcinoma. Hepatogastroenterology. 2008;55:1916–1920.
Dvory-Sobol H, Sagiv E, Liberman E, Kazanov D, Arber N. Suppression of gastric cancer cell growth by targeting the beta-catenin/T-cell factor pathway. Cancer. 2007;109:188–197.
Dvory-Sobol H, Sagiv E, Kazanov D, Ben-Ze’ev A, Arber N. Targeting the active beta-catenin pathway to treat cancer cells. Mol Cancer Ther. 2006;5:2861–2871.
de Belle I, Huang RP, Fan Y, Liu C, Mercola D, Adamson ED. p53 and Egr-1 additively suppress transformed growth in HT1080 cells but Egr-1 counteracts p53-dependent apoptosis. Oncogene. 1999;18:3633–3642.
Yu J, Zhang SS, Saito K, et al. PTEN regulation by Akt-EGR1-ARF-PTEN axis. EMBO J. 2009;28:21–33.
Kim J, Lee YH, Kwon TK, Chang JS, Chung KC, Min DS. Phospholipase D prevents etoposide-induced apoptosis by inhibiting the expression of early growth response-1 and phosphatase and tensin homologue deleted on chromosome 10. Cancer Res. 2006;66:784–793.
Mahalingam D, Natoni A, Keane M, Samali A, Szegezdi E. Early growth response-1 is a regulator of DR5-induced apoptosis in colon cancer cells. Br J Cancer. 2010;102:754–764.
Shtutman M, Zhurinsky J, Simcha I, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–5527.
Gnad T, Feoktistova M, Leverkus M, Lendeckel U, Naumann M. Helicobacter pylori-induced activation of beta-catenin involves low density lipoprotein receptor-related protein 6 and dishevelled. Mol Cancer. 2010;9:31.
Lee SH, Kang HJ, Shin DH, et al. Expression of beta-catenin and its mechanism of delocalization in intestinal-type early gastric cancer based on mucin expression. Histol Histopathol. 2009;24:831–838.
Saegusa M, Hashimura M, Kuwata T, et al. Transcription factor Egr1 acts as an upstream regulator of beta-catenin signalling through up-regulation of TCF4 and p300 expression during trans-differentiation of endometrial carcinoma cells. J Pathol. 2008;216:521–532.
Acknowledgments
We are grateful to Prof Chang-Woo Lee (Sungkyunkwan University School of Medicine, Korea) for the generous gift of the pcDNA3-Egr-1 plasmid used in this study. This work was supported by grants from the Specialized Research Fund for the Doctoral Program of Higher Education of China (20110073120105) and Shanghai Municipal Health Bureau foundation (20114169).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ting Sun and Hua Tian equally contributed to this work.
Rights and permissions
About this article
Cite this article
Sun, T., Tian, H., Feng, YG. et al. Egr-1 Promotes Cell Proliferation and Invasion by Increasing β-Catenin Expression in Gastric Cancer. Dig Dis Sci 58, 423–430 (2013). https://doi.org/10.1007/s10620-012-2356-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2356-4