Abstract
Background
We hypothesized that the severity of dextran sodium sulfate (DSS)-induced colitis could differ between DSS preparations of the same molecular weight, and that this difference may be affected by the sulfur content. To test this, we used three DSS preparations of similar molecular weights but with different sulfur contents.
Methods
Three DSS preparations with molecular weights of 40,000 to 50,000 were tested: MP Biomedicals (MP Bio), USB (USB), and The Lab Depot (The Lab). Epithelial cell lines were used to assess the levels of poly (ADP-ribose) polymerase (PARP) in the presence of 2.0% DSS in vitro. Eight-week-old female C57/B6 mice were fed 2.0% DSS in water for 1 week, and then sacrificed to investigate the effects of the DSS preparations in vivo.
Results
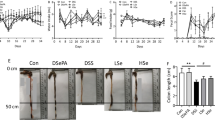
In vitro experiments using CaCo-2 and CMT-93 cells revealed decreased PARP levels from all DSS preparations. Notably, the PARP level was significantly decreased in CaCo-2 cells treated with DSS from USB as compared to The Lab Mice treated with The Lab DSS had significantly decreased body weight losses on day 7 as compared to mice receiving DSS from MP Bio and USB. This result was supported by their DAI score, colon weight/length ratio, and histological scores.
Conclusion
The severity of colitis can differ between similar DSS preparations of the same molecular weight range. This difference in colitogenic properties may be affected by the total sulfur content of each DSS preparation.









Similar content being viewed by others
References
Marcus R, Watt J. Seaweeds and ulcerative colitis in laboratory animals. Lancet. 1969;2:489–490.
Watt J, Marcus R. Ulceration of the colon in rabbits fed sulphated amylopectin. J Pharm Pharmacol. 1972;24:68–69.
Marcus R, Watt J. Ulcerative disease of the colon in laboratory animals induced by pepsin inhibitors. Gastroenterology. 1974;67:473–483.
Marcus R, Watt J. Colonic ulceration in guinea-pigs and rabbits fed lignosulphonate. Vet Rec. 1974;94:580.
Watt J, Marcus R. Proceedings: effect of lignosulphonate on the colon of guinea-pigs. Proc Nutr Soc. 1974;33:65A–66A.
Anderson W, Watt J. Inhibition of peptic activity, protection against histamine ulceration in the guinea pig, and combination with gastric mucin by an algal polyanion. J Pharm Pharmacol. 1959;11:318.
Barnes WA, Redo SF, Ecker RR, Wenig J. Dextran sulfate. A new and potent antiulcer agent. Am J Surg. 1967;113:27–31.
Vocac JA, Alphin RS. Effects and mechanism of action of a lignosulphonate on experimental gastric ulceration in rats. Eur J Pharmacol. 1968;4:99–102.
Rosen H, Townsend P, Seifter J. Effect of sodium polyanhydromannuronic acid sulfate on incidence of ulcers in the Shay rat. Proc Soc Exp Biol Med. 1956;92:439–440.
Bianchi RG, Cook DL. Antipeptic and antiulcerogenic properties of a synthetic sulfated polysaccharide (Sn-263). Gastroenterology. 1964;47:409–414.
Ohkusa T. Production of experimental ulcerative colitis in hamsters by dextran sulfate sodium and changes in intestinal microflora. Nihon Shokakibyo Gakkai Zasshi. 1985;82:1327–1336.
Melgar S, Karlsson A, Michaelsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol. 2005;288:G1328–G1338.
Hahm KB, Im YH, Parks TW, et al. Loss of transforming growth factor beta signalling in the intestine contributes to tissue injury in inflammatory bowel disease. Gut. 2001;49:190–198.
Araki Y, Mukaisho K, Sugihara H, Fujiyama Y, Hattori T. Proteus mirabilis sp. intestinal microflora grow in a dextran sulfate sodium-rich environment. Int J Mol Med. 2010;25:203–208.
Watt J, Marcus R. Experimental ulcerative disease of the colon in animals. Gut. 1973;14:506–510.
Araki Y, Mukaisyo K, Sugihara H, Fujiyama Y, Hattori T. Increased apoptosis and decreased proliferation of colonic epithelium in dextran sulfate sodium-induced colitis in mice. Oncol Rep. 2010;24:869–874.
Araki Y, Andoh A, Fujiyama Y, et al. Application of 2-aminopyridine fluorescence labeling in the analysis of in vivo and in vitro metabolism of dextran sulfate sodium by size-exclusion high-performance liquid chromatography. J Chromatogr. 2001;753:209–215.
Araki Y, Katoh T, Urabe M, Kishi Y, Ishizuka I, Fujiyama Y. The analysis of pyridylamino-dextran sulfate oligomers by high-performance liquid chromatography and a novel detection system for sulfated polysaccharides. Oncol Rep. 2004;12:363–367.
Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249.
Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–1652.
Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702.
Vlodavsky I, Miao HQ, Medalion B, Danagher P, Ron D. Involvement of heparan sulfate and related molecules in sequestration and growth promoting activity of fibroblast growth factor. Cancer Metastasis Rev. 1996;15:177–186.
Ohkusa T, Okayasu I, Tokoi S, Araki A, Ozaki Y. Changes in bacterial phagocytosis of macrophages in experimental ulcerative colitis. Digestion. 1995;56:159–164.
Axelsson LG, Landstrom E, Goldschmidt TJ, Gronberg A, Bylund-Fellenius AC. Dextran sulfate sodium (DSS) induced experimental colitis in immunodeficient mice: effects in CD4(+)-cell depleted, athymic and NK-cell depleted SCID mice. Inflamm Res. 1996;45:181–191.
Yu SW, Wang H, Poitras MF, et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bamba, S., Andoh, A., Ban, H. et al. The Severity of Dextran Sodium Sulfate-Induced Colitis Can Differ Between Dextran Sodium Sulfate Preparations of the Same Molecular Weight Range. Dig Dis Sci 57, 327–334 (2012). https://doi.org/10.1007/s10620-011-1881-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-011-1881-x