Abstract
Background and Aim
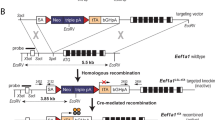
The regulation of human intestinal lactase-phlorizin hydrolase remains incompletely understood. One kb of pig and 2 kb of rat 5′-flanking sequence controls correct tissue, cell, topographic, and villus LCT expression. To gain insight into human LCT expression, transgenic mouse lines were generated from 3.3 kb of human LPH 5′ flanking sequence from a lactase persistent individual fused to a human growth hormone (hGH) reporter bounded by an insulator.
Methods
Four lines were identified in which reporter expression was specifically detectable in the intestine and no other organ, two of which demonstrated hGH expression specific to small and large intestine. Quantitative RT-PCR was carried out on proximal to distal segments of small intestine at fetal days 16.5 and 18.5 and at birth, postnatal days 7 and 28 in line 22.
Results
In fetal intestine, hGH expression demonstrated a proximal to distal gradient similar to that in native intestine. There was no significant difference between hGH expression levels at 7 and 28 days in segment 3, the midpoint of the small intestine, where expression of endogenous lactase is maximal at 7 days and declines significantly by 28 days. Distal small intestine displayed high levels of hGH expression in enteroendocrine cells, which were shown to be a subset of the PYY cells.
Conclusions
Thus, a 3.3-kb LPH 5′ flanking sequence construct from a lactase persistent individual is able to maintain postnatal expression in transgenic mice post weaning.







Similar content being viewed by others
References
Troelsen JT. Adult-type hypolactasia and regulation of lactase expression. Biochim Biophys Acta. 2005;1723(1–3):19–32.
Krasinski SD, Estrada G, Yeh KY, et al. Transcriptional regulation of intestinal hydrolase biosynthesis during postnatal development in rats. Am J Physiol. 1994;267(4 Pt 1):G584–G594.
Rings EH, Krasinski SD, van Beers EH, et al. Restriction of lactase gene expression along the proximal-to-distal axis of rat small intestine occurs during postnatal development. Gastroenterology. 1994;106(5):1223–1232.
Montgomery RK, Büller HA, Rings EH, Grand RJ. Lactose intolerance and the genetic regulation of intestinal lactase-phlorizin hydrolase. Faseb J. 1991;5(13):2824–2832.
Sahi T, Isokoski M, Jussila J, et al. Recessive inheritance of adult-type lactose malabsorption. Lancet. 1973;2(7833):823–826.
Boll W, Wagner P, Mantei N. Structure of the chromosomal gene and cDNAs coding for lactase-phlorizin hydrolase in humans with adult-type hypolactasia or persistence of lactase. Am J Hum Genet. 1991;48(5):889–902.21.
Tishkoff SA, Reed FA, Ranciaro A, et al. Convergent adaptation of human lactase persistence in Africa and Europe. Nat Genet. 2007;39(1):31–40.
Bersaglieri T, Sabeti PC, Patterson N, et al. Genetic signatures of strong recent positive selection at the lactase gene. Am J Hum Genet. 2004;74(6):1111–1120.
van Wering HM, Bosse T, Musters A, et al. Complex regulation of the lactase-phlorizin hydrolase promoter by GATA-4. Am J Physiol Gastrointest Liver Physiol. 2004;287(4):G899–G909.
Grand RJ, Montgomery RK, Chitkara DK, Hirschhorn JN. Changing genes; losing lactase. Gut. 2003;52(5):617–619.
Troelsen JT, Mehlum A, Olsen J, et al. 1 kb of the lactase-phlorizin hydrolase promoter directs post-weaning decline and small intestinal-specific expression in transgenic mice. FEBS Lett. 1994;342(3):291–296.
Krasinski SD, Upchurch BH, Irons SJ, et al. Rat lactase-phlorizin hydrolase/human growth hormone transgene is expressed on small intestinal villi in transgenic mice. Gastroenterology. 1997;113(3):844–855.
Lee SY, Wang Z, Lin CK, et al. Regulation of intestine-specific spatiotemporal expression by the rat lactase promoter. J Biol Chem. 2002;277(15):13099–13105.
Verhave M, Krasinski SD, Christian SI, et al. Regulatory regions in the rat lactase-phlorizin hydrolase gene that control cell-specific expression. J Pediatr Gastroenterol Nutr. 2004;39(3):275–285.
Bosse T, van Wering HM, Gielen M, et al. Hepatocyte nuclear factor-1alpha is required for expression but dispensable for histone acetylation of the lactase-phlorizin hydrolase gene in vivo. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G1016–G1024.
Bonifer C, Vidal M, Grosveld F, Sippel AE. Tissue-specific and position-independent expression of the complete gene domain for chicken lysozyme in transgenic mice. Embo J. 1990;9(9):2843–2848.
Burgess-Beusse B, Farrell C, Gaszner M, et al. The insulation of genes from external enhancers and silencing chromatin. Proc Natl Acad Sci USA. 2002;99(Suppl 4):16433–16437.
Calvert R, Micheletti PA. Selection of a chemically defined medium for culturing fetal mouse small intestine. In Vitro. 1981;17(4):331–344.
Böttcher G, Sjölund K, Ekblad E, Håkanson R, Schwartz TW, Sundler F. Coexistence of peptide YY and glicentin immunoreactivity in endocrine cells of the gut. Regul Pept. 1984;8(4):261–266.
Olds LC, Sibley E. Lactase persistence DNA variant enhances lactase promoter activity in vitro: functional role as a cis regulatory element. Hum Mol Genet. 2003;12(18):2333–2340.
Markowitz AJ, Wu GD, Bader A, Cui Z, Chen L, Traber PG. Regulation of lineage-specific transcription of the sucrase-isomaltase gene in transgenic mice and cell lines. Am J Physiol. 1995;269(6 Pt 1):G925–G939.
Sweetser DA, Birkenmeier EH, Hoppe PC, McKeel DW, Gordon JI. Mechanisms underlying generation of gradients in gene expression within the intestine: an analysis using transgenic mice containing fatty acid binding protein-human growth hormone fusion genes. Genes Dev. 1988;2(10):1318–1332.
Simon TC, Roth KA, Gordon JI. Use of transgenic mice to map cis-acting elements in the liver fatty acid-binding protein gene (Fabpl) that regulate its cell lineage-specific, differentiation-dependent, and spatial patterns of expression in the gut epithelium and in the liver acinus. J Biol Chem. 1993;268(24):18345–18358.
Jenny M, Uhl C, Roche C, et al. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. Embo J. 2002;21(23):6338–6347.
Zhou J, Hegsted M, Mccutcheon KL, et al. Peptide YY and proglucagon mRNA expression patterns and regulation in the gut. Obesity (Silver Spring). 2006;14(4):683–689.
Enattah NS, Sahi T, Savilahti E, Terwilliger JD, Peltonen L, Järvelä I. Identification of a variant associated with adult-type hypolactasia. Nat Genet. 2002;30(2):233–237.
Acknowledgments
The authors are indebted to Stuart Orkin, MD, for generous provision of the insulator, Andrew B. Leiter, PhD for provision of the PYY antibody, and to Christina Piaseckyj for superb technical support. Transgenic mice were generated in the Center of Excellence for Molecular Hematology supported by a grant from the NIDDK (DK49216) to Stuart H. Orkin. Supported in part by NIH MERIT Award DK R37 32658, NIH Research Grant R01 DK 061382, and the Harvard Digestive Disease Center P30 DK 34854.
Conflict of interest
None of the authors has a conflict of interest of any kind.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nana Yaa Baffour-Awuah and Eveline Delemarre contributed equally to this research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental figure 1
. Representation of the 3.3-kb human LPH construct used in this study (see Methods for details). Supplemental figure 2. The transgene does not significantly affect the weight of line 22 mice. Transgenic mice were weighed weekly and compared to their hGH-negative litter mates. Weight is plotted in grams with standard deviations shown for wt male (purple), L22 male (red) and L22 female (green). (PDF 77 kb)
Rights and permissions
About this article
Cite this article
Baffour-Awuah, N.Y., Delemarre, E., Fujiwara, Y. et al. Characterization of Expression in Mice of a Transgene Containing 3.3 kb of the Human Lactase-Phlorizin Hydrolase (LPH) 5′ Flanking Sequence. Dig Dis Sci 56, 59–69 (2011). https://doi.org/10.1007/s10620-010-1480-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-010-1480-2