Abstract
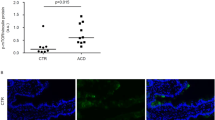
Background Aberrant signaling via the p21/mitogen-activated proteins (MAP) kinase pathway has been described in lymphocytes of patients with various autoimmune diseases. There is little published data about the intracellular mediators and signals that regulate expression and activity of transcription factors and their effect on celiac disease induction and progression. Aim To investigate the possible involvement of MAP kinase pathway in peripheral blood mononuclear cells (PBMC) in celiac disease and its correlation with disease activity. Methods Expression of the total and activated forms of two MAP kinases [extracellular response kinase (ERK) and c-Jun amino terminal kinase (JNK)] were studied by Western blots in PBMC of 17 untreated and 19 treated celiac patients, and 17 controls. Seven of these untreated celiac patients were studies before and after 6 months of gluten-free diet. Results Phosphorylated ERK of active celiac disease patients was significantly lower compared with controls (P < 0.01) and was increased towards normal after 6 month of gluten-free diet (P < 0.01). Phosphorylated JNK was increased significantly in the untreated celiac group (P < 0.01) and normalized towards the control level after 6 months of gluten-free diet (P < 0.04). Conclusions Aberrant MAP-kinase pathway activity is associated with active celiac disease (CD). Further studies should examine the potential role of this aberration in pathogenesis of CD.



Similar content being viewed by others
References
National Institute of Health Consensus Development Conference Statement on Celiac Disease, 2004. Gastroenterology. 2005;128(suppl):S1–S9.
Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180–188. doi:10.1056/NEJMra010852.
Kagnoff MF. Coeliac disease: genetic immunological and environmental factors in disease pathogenesis. Scand J Gastroenterol. 1985;114(suppl):45–54. doi:10.3109/00365528509093767.
Kagnoff MF. Immunopathogenesis of celiac disease. Immunol Invest. 1989;18:499–508. doi:10.3109/08820138909112259.
Kagnoff MF. Understanding the molecular basis of celiac disease. Gut. 1990;31:497–499. doi:10.1136/gut.31.5.497.
Nalual AT, Nilsson S, Gudjonsdottir AH, et al. Genome-wide linkage analysis of Scandinavian affected sib-pairs supports presence of susceptibility loci for celiac disease on chromosome 5 and 11. Eur J Hum Genet. 2001;9:938–944. doi:10.1038/sj.ejhg.5200752.
Rincon M, Flavell RA, Davis RA. The JNK and P38 MAP kinase signaling pathway in T cell-mediated immune responses. Free Radic Biol Med. 2000;28:1328–1337. doi:10.1016/S0891-5849(00)00219-7.
Zhang YL, Dong C. MAP kinases in immune respon. Cell Mol Immunol. 2005;2:20–27.
Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi:10.1146/annurev.immunol.20.091301.131133.
Rapoport MJ, Lazarus AH, Jaramillo A, et al. Thymic T cell anergy in autoimmune non-obese diabetic mice is mediated by deficient T cell receptor regulation of the pathway of p21ras activation. J Exp Med. 1993;177:1221–1226. doi:10.1084/jem.177.4.1221.
Gross DJ, Sidi H, Weiss A, et al. Prevention of diabetes mellitus in non-obese diabetic mice by Linomide-a novel immunomodulator drug. Diabetologia. 1994;37:1195–1201. doi:10.1007/BF00399792.
Rapoport MJ, Weiss L, Mor A, et al. Prevention of autoimmune diabetes by Linomide in NOD mice is associated with upregulation of the T cell receptor mediated activation of p21ras. J Immunol. 1996;157:4721–4725.
Salojin K, Zhang J, Cameron M, et al. Impaired plasma membrane targeting of Grb2-murine son of sevenless (mSOS) complex and differential activation of the Fyn-T cell receptor (TCR)-ζ-Cbl pathway mediate T cell hyporesponsiveness in autoimmune nonobese diabetic mice. J Exp Med. 1997;186:887–897. doi:10.1084/jem.186.6.887.
Rapoport MJ, Amit M, Aharoni D, et al. Constitutive up-regulated activity of MAP kinase is associated with down regulated early p21ras pathway in lymphocytes of SLE patients. JAI. 2002;19:63–70.
Rapoport MJ, Mor A, Amit M, et al. Decreased expression of the p21 ras stimulatory factor hSOS in PBMC from inactive SLE patients. Lupus. 1999;8:24–28. doi:10.1191/096120399678847362.
Waetzig GH, Seegert D, Rosenstiel P, et al. p38 mitogen activated protein kinase is activated and linked to TNF-α signaling in inflammatory bowel disease. J Immunol. 2002;168:5342–5351.
van Nontfrans C, Peppelenbosch M, te Veld AA, et al. Inflammatory signal transduction in Crohn’s disease and novel therapeutic approaches. Biochem Pharmacol. 2002;64:789–795.
Hommes D, Van den Blink B, Plasse T, et al. Inhibition of stress-activated MAP kinases induces clinical improvement in moderate to severe Crohn’s disease. Gastroenterology. 2002;122:7–14.
Van den Blink B, Ten Hovve T, Van den Brink GR. From extracellular targets, inhibitng MAP kinases in treatment of Crohn’s disease. Ann NY Acad Sci. 2002;973:349–358.
Confino-Cohen R, Aharoni D, Goldberg A, et al. Evidence for aberrant regulation of the p21ras pathway in PBMCs of patients with chronic idiopathic urticaria. J Allergy Clin Immunol. 2002;109:349–356.
Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654.
Dewar DH, Ciclitira PJ. Clinical features and diagnosis of celiac disease. Gastroenterology. 2005;128(suppl):S19–S24.
Hill ID. What are the sensitivity and specificity of serologic test for celiac disease? Do sensitivity and specifity vary in different populations? Gastroenterology. 2005;128(suppl):S25–S32.
Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: a conserved switch to diverse cell function. Nature. 1990;348:125–131.
Izquiero PM, Reif K, Cantrell D. The regulation and function of p21ras during T-cell activation and growth. Immunol Today. 1995;16:672–676.
Finkel TH, Marrack P, Kappler JW, et al. Alpha beta T cell receptor and CD3 transduce different signals in immature T cells. Implications for selection and tolerance. J Immunol. 1989;142:3006–3012.
Marrack P, Kappler J, Kotzin BL. Autoimmune disease: why and where it occurs. Nature Med. 2001;7:899–905.
Jelikova LP, Rozkova D, Pecharova B, et al. Gliadin fragments induce phenotypic and functional maturation of human dendritic cells. J Immunol. 2005;175:7038–7045.
Di Sabatino A, D’Alo S, Millimaggi D, et al. Apoptosis and peripheral blood lymphocyte depletion in celiac disease. Immunology. 2001;103:435–440.
Maiuri L, Clacci C, Ricciardelli I, et al. Association between innate respons to gliadin and activation of pathogenic T cells in coeliac disease. Lancet. 2003;362:30–37.
Matsumoto N, Imamura R, Suda T. Caspase-8-and JNK-dependent AP-1 activation is required for FAS ligand-induced IL-8 production. FEBS J. 2007;274:2376–2384.
Pahl A, Zhang M, Kuss H, et al. Role of TCR-induced extracellular signal-regulated kinase activation in the regulation of early IL-4 expression in naive CD4+ T cells. J Iummunol. 2003;170:2427–2434.
Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic Autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269.
Steinberg AD, Klinman DM, Kastner DL, et al. Genetic and molecular genetic studies of murine and human lupus. J Rheumatol. 1987;14(suppl):166–176.
Rapoport MJ, Mor A, Vardi P, et al. Defective activation of p21ras in peripheral blood mononuclear cells from patients with insulin dependent diabetes mellitus. Autoimmunity. 1999;29:147–154.
Sharabani A, Haviv A, Zinger H, Dayan M, Mozes E. Amelioration of murine lupus by a peptide based on the complemtarity determining region-1 of an autoantibody as compared to dexamethasone. Different effects on cytokines and apoptosis. Immunology. 2007;121:248–257.
Author information
Authors and Affiliations
Corresponding author
Additional information
E. Broide MD and E. Scapa MD have contributed equally to this paper and therefore should be considered first authors.
Rights and permissions
About this article
Cite this article
Broide, E., Scapa, E., Bloch, O. et al. Evidence for Aberrant Regulation of MAP Kinase Signal Transduction Pathway in Peripheral Blood Mononuclear Cells in Patients with Active Celiac Disease. Dig Dis Sci 54, 1270–1275 (2009). https://doi.org/10.1007/s10620-008-0480-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-008-0480-y