Abstract
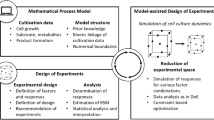
In Chinese Hamster Ovary (CHO) cell lines, the establishment of the ideal fed-batch regimen promotes metabolic conditions advantageous for the bioproduction of therapeutic molecules. A tailored, cell line-specific feeding scheme is typically defined during process development (PD) activities, through the incorporation of Design of Experiment (DOE) and late stage cell culture approaches. The feeding during early stage cell line development (CLD) was a simplified “one-fits-all” design, inherited from PD lab, that didn’t account for CLD needs of throughput and streamlined workflow. The “one-fits-all” efficiency was not routinely verified when novel technologies were incorporated in CLD and sub-optimal feeding carried the risk of not selecting the most desirable cell lines amenable to late stage PD. In our work we developed the DOE-feed method; a streamlined, three-stages framework for identifying efficient feeding schemes as the CLD technologies evolved. We combined early stage cell culture input data with late-stage techniques, such as statistical modelling, principal component analysis (PCA), DOE and Prediction Profiler. Novel in our DOE-feed work, we deliberately anticipated the application of statistics and approached the method development as an early-stage, continuously updated process, by building iterative datasets and statistically interpreting their responses. We capitalized on the statistical models defined by the DOE-feed methodology to study the influence of feeds on daily productivity and growth and to extrapolate feeding-schemes that improved the cell line screening. The DOE-feed became a methodology suited for CLD needs at AbbVie, and optimized the early stage screening, reduced the operational hands-on time and improved the overall workstream efficiency.








Similar content being viewed by others
References
Abu-Absi SF, Yang L, Thompson P, Jiang C, Kandula S, Schilling B, Shukla AA (2010) Defining process design space for monoclonal antibody cell culture. Biotechnol Bioeng 106:894–905. https://doi.org/10.1002/bit.22764
Alves CS, Gilbert A, Dalvi S, Germain BS, Xie W, Estes S, Kshirsagar R, Ryll T (2015) Integration of cell line and process development to overcome the challenge of a difficult to express protein. Biotechnol Prog 31:1201–1211
Ben Yahia B, Malphettes L, Heinzle E (2015) Macroscopic modeling of mammalian cell growth and metabolism. Appl Microbiol Biotechnol 99:7009–7024. https://doi.org/10.1007/s00253-015-6743-6
Berry B, Moretto J, Matthews T, Smelko JWK, Berry B, Moretto J, Matthews T, Smelko J, Wiltberger K, Berry B, Moretto J, Matthews T, Smelko JWK (2014) Cross-scale predictive modeling of CHO cell culture growth and metabolites using Raman spectroscopy and multivariate analysis. Biotechnol Prog. https://doi.org/10.1002/btpr.2035
Brühlmann D, Jordan M, Hemberger J, Sauer M, Stettler M, Broly H (2015) Tailoring recombinant protein quality by rational media design. Biotechnol Prog 31:615–629
Brühlmann D, Sokolov M, Butté A, Sauer M, Hemberger J, Souquet J, Broly H, Jordan M (2017) Parallel experimental design and multivariate analysis provides efficient screening of cell culture media supplements to improve biosimilar product quality. Biotechnol Bioeng 114:1448–1458
Butler M, Meneses-Acosta A (2012) Recent advances in technology supporting biopharmaceutical production from mammalian cells. Appl Microbiol Biotechnol 96:885–894
Charaniya S, Le H, Rangwala H, Mills K, Johnson K, Karypis G, Hu W-S (2010) Mining manufacturing data for discovery of high productivity process characteristics. J Biotechnol 147:186–197
Chusainow J, Yang YS, Yeo JHM, Ton PC, Asvadi P, Wong NSC, Yap MGS (2009) A study of monoclonal antibody-producing CHO cell lines: what makes a stable high producer? Biotechnol Bioeng 102:1182–1196
Davies SL, O’Callaghan PM, Mcleod J, Pybus LP, Sung YH, Rance J, Wilkinson SJ, Racher AJ, Young RJ, James DC (2011) Impact of gene vector design on the control of recombinant monoclonal antibody production by Chinese hamster ovary cells. Biotechnol Prog 27:1689–1699
Dietmair S, Hodson MP, Quek LE, Timmins NE, Chrysanthopoulos P, Jacob SS, Gray P, Nielsen LK (2012) Metabolite profiling of CHO cells with different growth characteristics. Biotechnol Bioeng 109:1404–1414
Dutton RL, Scharer J, Moo-Young M (2006) Cell cycle phase dependent productivity of a recombinant Chinese hamster ovary cell line. Cytotechnology 52:55–69
Estes S, Melville M (2014) Mammalian cell line developments in speed and efficiency. Adv Biochem Eng Biotechnol 123:127–141
Galleguillos SN, Ruckerbauer D, Gerstl MP, Borth N, Hanscho M, Zanghellini J (2017) What can mathematical modelling say about CHO metabolism and protein glycosylation? Comput Struct Biotechnol J 15:212–221
Gauthier JH, Pohl PI (2011) A general framework for modeling growth and division of mammalian cells. BMC Syst Biol 5:3
Hiller GW, Ovalle AM, Gagnon MP, Curran ML, Wang W (2017) Cell-controlled hybrid perfusion fed-batch CHO cell process provides significant productivity improvement over conventional fed-batch cultures. Biotechnol Bioeng 114:1438–1447
Ho SCL, Yang Y (2014) Identifying and engineering promoters for high level and sustainable therapeutic recombinant protein production in cultured mammalian cells. Biotechnol Lett 36:1569–1579
Huang Y-M, Hu W, Rustandi E, Chang K, Yusuf-Makagiansar H, Ryll T (2010) Maximizing productivity of CHO cell-based fed-batch culture using chemically defined media conditions and typical manufacturing equipment. Biotechnol Prog 26:1400–1410
Kang S, Ren D, Xiao G, Daris K, Buck L, Enyenihi AA, Zubarev R, Bondarenko PV, Deshpande R (2014) Cell line profiling to improve monoclonal antibody production. Biotechnol Bioeng 111:748–760
Khattak SF, Xing Z, Kenty B, Koyrakh I, Li ZJ (2010) Feed development for fed-batch CHO production process by semisteady state analysis. Biotechnol Prog 26:797–804
Kim JY, Kim YG, Lee GM (2012) CHO cells in biotechnology for production of recombinant proteins: current state and further potential. Appl Microbiol Biotechnol 93:917–930
Kumar N, Gammell P, Clynes M (2007) Proliferation control strategies to improve productivity and survival during CHO based production culture: a summary of recent methods employed and the effects of proliferation control in product secreting CHO cell lines. Cytotechnology 53:33–46
Kyriakopoulos S, Kontoravdi C (2014) A framework for the systematic design of fed-batch strategies in mammalian cell culture. Biotechnol Bioeng 111:1–25
Lindgren K, Salmén A, Lundgren M, Bylund L, Ebler A, Fäldt E, Sörvik L, Fenge C, Skoging-Nyberg U (2009) Automation of cell line development. Cytotechnology 59:1–10. https://doi.org/10.1007/s10616-009-9187-y
Lloyd DR, Leelavatcharamas V, Emery AN, Al-Rubeai M (1999) The role of the cell cycle in determining gene expression and productivity in CHO cells. Cytotechnology 30:49–57
Lu F, Toh PC, Burnett I, Li F, Hudson T, Amanullah A, Li J (2013) Automated dynamic fed-batch process and media optimization for high productivity cell culture process development. Biotechnol Bioeng 110:191–205
Mcleod J, O’Callaghan PM, Pybus LP, Wilkinson SJ, Root T, Racher AJ, James DC (2011) An empirical modeling platform to evaluate the relative control discrete CHO cell synthetic processes exert over recombinant monoclonal antibody production process titer. Biotechnol Bioeng 108:2193–2204
Mora A, Zhang SS, Carson G, Nabiswa B, Hossler P, Yoon S (2017) Sustaining an efficient and effective CHO cell line development platform by incorporation of 24-deep well plate screening and multivariate analysis. Biotechnol Prog. https://doi.org/10.1002/btpr.2584
Nagashima H, Watari A, Shinoda Y, Okamoto H, Takuma S (2013) Application of a Quality by Design approach to the cell culture process of monoclonal antibody production, resulting in the establishment of a Design space. J Pharm Sci 102:4274–4283
Nolan RP, Lee K (2012) Dynamic model for CHO cell engineering. J Biotechnol 158:24–33. https://doi.org/10.1016/j.jbiotec.2012.01.009
O’Callaghan PM, McLeod J, Pybus LP, Lovelady CS, Wilkinson SJ, Racher AJ, Porter A, James DC (2010) Cell line-specific control of recombinant monoclonal antibody production by CHO cells. Biotechnol Bioeng 106:938–951
Porter AJ, Racher AJ, Preziosi R, Dickson AJ (2010) Strategies for selecting recombinant CHO cell lines for cGMP manufacturing: improving the efficiency of cell line generation. Biotechnol Prog 26:1455–1464
Povey JF, O’Malley CJ, Root T, Martin EB, Montague GA, Feary M, Trim C, Lang DA, Alldread R, Racher AJ, Smales CM (2014) Rapid high-throughput characterisation, classification and selection of recombinant mammalian cell line phenotypes using intact cell MALDI-ToF mass spectrometry fingerprinting and PLS-DA modelling. J Biotechnol 184:84–93
Rouiller Y, Solacroup T, Deparis V, Barbafieri M, Gleixner R, Broly H, Eon-Duval A (2012) Application of quality by design to the characterization of the cell culture process of an Fc-Fusion protein. Eur J Pharm Biopharm 81:426–437. https://doi.org/10.1016/j.ejpb.2012.02.018
Rouiller Y, Périlleux A, Collet N, Jordan M, Stettler M, Broly H (2013) A high-throughput media design approach for high performance mammalian fed-batch cultures. MAbs 5:501–511
Saito H, Posas F (2012) Response to hyperosmotic stress. Genetics 192:289–318
Scarcelli JJ, Shang TQ, Iskra T, Allen MJ, Zhang L (2017) Strategic deployment of CHO expression platforms to deliver Pfizer’s Monoclonal Antibody Portfolio. Biotechnol Prog. https://doi.org/10.1002/btpr.2493
Sellick CA, Croxford AS, Maqsood AR, Stephens G, Westerhoff HV, Goodacre R, Dickson AJ (2011) Metabolite profiling of recombinant CHO cells: designing tailored feeding regimes that enhance recombinant antibody production. Biotechnol Bioeng 108:3025–3031
Seth G, Charaniya S, Wlaschin KF, Hu WS (2007) In pursuit of a super producer-alternative paths to high producing recombinant mammalian cells. Curr Opin Biotechnol 18:557–564
Shirsat N, Mohd A, Whelan J, English NJ, Glennon B, Al-Rubeai M (2015) Revisiting Verhulst and Monod models: analysis of batch and fed-batch cultures. Cytotechnology 67:515–530
Templeton N, Dean J, Reddy P, Young JD (2013) Peak antibody production is associated with increased oxidative metabolism in an industrially relevant fed-batch CHO cell culture. Biotechnol Bioeng 110:2013–2024
Tsang VL, Wang AX, Yusuf-Makagiansar H, Ryll T (2013) Development of a scale down cell culture model using multivariate analysis as a qualification tool. Biotechnol Prog 30:152–160
Yang WC, Minkler DF, Kshirsagar R, Ryll T, Huang YM (2016) Concentrated fed-batch cell culture increases manufacturing capacity without additional volumetric capacity. J Biotechnol 217:1–11
Ye J, Alvin K, Latif H, Hsu A, Parikh V, Whitmer T, Tellers M, de la Cruz Edmonds MC, Ly J, Salmon P, Markusen JF (2010) Rapid protein production using CHO stable transfection pools. Biotechnol Prog 26:1431–1437. https://doi.org/10.1002/btpr.469
Yu M, Hu Z, Pacis E, Vijayasankaran N, Shen A, Li F (2011) Understanding the intracellular effect of enhanced nutrient feeding toward high titer antibody production process. Biotechnol Bioeng 108:1078–1088
Zhang H, Wang H, Liu M, Zhang T, Zhang J, Wang X, Xiang W (2013) Rational development of a serum-free medium and fed-batch process for a GS-CHO cell line expressing recombinant antibody. Cytotechnology 65:363–378
Zhang A, Tsang VL, Moore B, Shen V, Huang Y-M, Kshirsagar R, Ryll T (2015) Advanced process monitoring and feedback control to enhance cell culture process production and robustness. Biotechnol Bioeng 112:2495–2504
Funding
The design, study conduct, and financial support for the study were provided by AbbVie.
Author information
Authors and Affiliations
Contributions
The authors participated in the interpretation of data, review, and approval of the publication; all authors contributed to the development of the publication and maintained control over the final content. AM, BN, YY, SZ, and GC have or had a financial interest in AbbVie. BN, YY and GC are AbbVie employees. AM and SZ are former employees of AbbVie. SY serves as a PhD advisor to AM.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mora, A., Nabiswa, B., Duan, Y. et al. Early integration of Design of Experiment (DOE) and multivariate statistics identifies feeding regimens suitable for CHO cell line development and screening. Cytotechnology 71, 1137–1153 (2019). https://doi.org/10.1007/s10616-019-00350-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-019-00350-1