Abstract
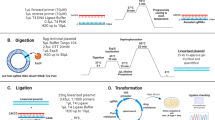
The clustered regulatory interspersed short palindromic repeat (CRISPR)/CRISPR-associated protein (Cas) system has been widely used for gene knock-out. Lentiviral vectors have been commonly used as a delivery method for this system, however, prolonged Cas9/sgRNA expression due to lentiviral integration can lead to accumulating off-target mutations. To solve this issue in engineering a gene knock-out cell line, this study established a novel system, which was composed of two lentiviral vectors. One lentiviral vector carried simultaneously sgRNAs and CRISPR/Cas9 expression cassettes targeting single or multiple gene(s); the other lentiviral vector carried Cre that could remove excess sgRNAs and Cas9 expression cassettes in the genome after gene targeting was achieved. To prove the principle, two candidate genes, extracellular matrix protein 1 (ECM1) and progranulin (PGRN), both highly expressed in MDA-MB-231 cells, were selected for testing the novel system. A dual knock-out of ECM1 and PGRN was successfully achieved in MDA-MB-231 cell line, with the sgRNAs and Cas9 expression cassettes being removed by Cre. This system should have great potential in applications for multiple genes knock-out in vitro.




Similar content being viewed by others
References
Auer TO, Duroure K, De CA, Concordet JP, Del BF (2013) Highly efficient crispr/cas9-mediated knock-in in zebrafish by homology-independent dna repair. Genome Res 24:142–153
Chai L, Mao Q, Liu S, Xia H (2011) Domain-specific monoclonal antibodies produced against human PGRN. Hybridoma 30:271–278
Chang J, Tang L, Lei H, Zhang XG, Zuo Z, Huang W, Fu H (2014) Effects of lentiviral infection of mesenchymal stem cells on the expression of octamer transcription factor 4. Mol Med Rep 10:2249–2254
Chen H, Jia WD, Li JS, Wang W, Xu GL, Ma JL, Ren WH, Ge YS, Yu JH, Liu WB, Zhang CH, Wang YC (2011) Extracellular matrix protein 1, a novel prognostic factor, is associated with metastatic potential of hepatocellular carcinoma. Med Oncol 28:S318–S325
Cheng R, Peng J, Yan Y, Cao P, Wang J, Qiu C, Tang L, Liu D, Tang L, Jin J, Huang X, He F, Zhang P (2014) Efficient gene editing in adult mouse livers via adenoviral delivery of CRISPR/Cas9. FEBS Lett 588:3954–3958
Cheung ST, Wong SY, Leung KL, Chen X, So S, Ng IO, TatFan S (2004) Granulin-epithelin precursor overexpression promotes growth and invasion of hepatocellular carcinoma. Clin Cancer Res 10:7629–7636
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823
Dang Y, Jia G, Choi J, Ma H, Anaya E, Ye C, Shankar P, Wu H (2015) Optimizing sgRNA structure to improve CRISPR-Cas9 knockout efficiency. Genome Biol 16:280–289
Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD (2013) High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol 31:822–826
Fu Y, Sander JD, Reyon D, Cascio VM, Joung JK (2014) Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol 32:279–284
Fujii W, Kawasaki K, Sugiura K, Naito K (2013) Efficient generation of large-scale genome-modified mice using gRNA and CAS9 endonuclease. Nucleic Acids Res 41:e187
González F, Zhu Z, Shi ZD, Lelli K, Verma N, Li QV, Huangfu D (2014) An icrispr platform for rapid, multiplexable, and inducible genome editing in human pluripo tent stem cells. Cell Stem Cell 15:215–226
Han X, Liu Z, Jo MC, Zhang K, Li Y, Zeng Z, Li N, Zu Y, Qin L (2015) CRISPR-Cas9 delivery to hard-to-transfect cells via membrane deformation. Sci Adv 1:e1500454
Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, Cradick TJ, Marraffini LA, Bao G, Zhang F (2013) DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 31:827–832
Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J (2013) RNA-programmed genome editing in human cells. Elife 2:e00471
Joseph A, Zheng JH, Chen K, Dutta M, Chen C, Stiegler G, Kunert R, Follenzi A, Goldstein H (2010) Inhibition of in vivo HIV infection in humanized mice by gene therapy of human hematopoietic stem cells with a lentiviral vector encoding a broadly neutralizing anti-hiv antibody. J Virol 84:6645–6653
Kim VN, Mitrophanous K, Kingsman SM, Kingsman AJ (1998) Minimal requirement for a lentivirus vector based on human immunodeficiency virus type 1. J Virol 72:811–816
Kim S, Kim D, Cho SW, Kim J, Kim JS (2014) Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res 24:1012–1019
Kong L, Tian Q, Guo F, Mucignat MT, Perris R, Sercu S, Merregaert J, Di Cesare PE, Liu C (2010) Interaction between cartilage oligomeric matrix protein and extracellular matrix protein 1 mediates endochondral bone growth. Matrix Biol 29:276–286
Lee K, Nam K, Oh S, Lim J, Kim Y, Lee JW, Yu J, Ahn S, Kim S, Noh D, Lee T, Shin I (2014) Extracellular matrix protein 1 regulates cell proliferation and trastuzumab resistance through activation of epidermal growth factor-signaling. Breast Cancer Res 16:479–495
Li MJ, Kim J, Li S, Zaia J, Yee JK, Anderson J, Akkina R, Rossi JJ (2005) Long-term inhibition of HIV-1 infection in primary hematopoietic cells by lentiviral vector delivery of a triple combination of anti-hiv shrna, anti-ccr5 ribozyme, and a nucleolar-localizing tar decoy. Mol Ther 12:900–909
Li S, Xue H, Wu J, Rao MS, Kim DH, Deng W, Liu Y (2015) Human induced pluripotent stem cell NEUROG2 dual knockin reporter lines generated by the CRISPR/Cas9 System. Stem Cells Dev 24:2925–2942
Li Y, Li Y, Zhao J, Wang D, Mao Q, Xia H (2016) Production and characterization of domain-specific monoclonal antibodies against human ECM1. Protein Expres Purif 121:103–111
Liao J, Karnik R, Gu H, Ziller MJ, Clement K, Tsankov AM, Akopian V, Gifford CA, Donaghey J, Galonska C, Pop R, Reyon D, Tsai SQ, Mallard W, Joung JK, Rinn JL, Gnirke A, Meissner A (2015) Targeted disruption of dnmt1, dnmt3a and dnmt3b in human embryonic stem cells. Nat Genet 47:469–478
Lin S, Staahl BT, Alla RK, Doudna JA (2014a) Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. Elife 3:e04766
Lin Y, Cradick TJ, Brown MT, Deshmukh H, Ranjan P, Sarode N, Wile BM, Vertino PM, Stewart FJ, Bao G (2014b) CRISPR/Cas9 systems have off-target activity with insertions or deletions between target DNA and guide RNA sequences. Nucleic Acids Res 42:7473–7485
Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM (2013) RNA-Guided human genome engineering via Cas9. Science 339:823–826
Moore R, Spinhirne A, Lai MJ, Preisser S, Li Y, Kang T, Bleris L (2014) CRISPR-based self-cleaving mechanism for controllable gene delivery in human cells. Nucleic Acids Res 43:1297–1303
Perez EE, Riley JL, Carroll RG, Von LD, June CH (2005) Suppression of HIV-1 infection in primary CD4 T cells transduced with a self-inactivating lentiviral vector encoding a membrane expressed gp41-derived fusion inhibitor. Clin Immunol 115:26–32
Qin XF, An DS, Chen IS, Baltimore D (2003) Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering rna against ccr5. Proc Natl Acad Sci U S A 100:183–188
Rahdara M, McMahon MA, Prakasha TP, Swayzea EE, Bennetta CF, Cleveland DW (2015) Synthetic CRISPR RNA-Cas9-guided genome editing in human cells. Proc Natl Acad Sci U S A 112:E7110–E7117
Ramezani A, Hawley TS, Hawley RG (2000) Lentiviral vectors for enhanced gene expression in human hematopoietic cells. Mol Ther 2:458–469
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F (2013) Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8:2281–2308
Semple-Rowland SL, Berry J (2014) Use of lentiviral vectors to deliver and express bicistronic transgenes in developing chicken embryos. Methods 66:466–473
Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG, Zhang F (2014) Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343:84–87
Song Y, Yuan L, Wang Y, Chen M, Deng J, Lv Q, Sui T, Li Z, Lai L (2016) Efficient dual sgrna-directed large gene deletion in rabbit with crispr/cas9 system. Cell Mol Life Sci 73:1–10
Sung YH, Kim JM, Kim HT, Lee J, Jeon J, Jin Y, Choi JH, Ban YH, Ha SJ, Kim CH, Lee HW, Kim JS (2014) Highly efficient gene knockout in mice and zebrafish with rna-guided endonucleases. Genome Res 24:125–131
Tangkeangsirisin W, Serrero G (2004) PC cell-derived growth factor (PCDGF/GP88) stimulates migration, invasiveness and VEGF expression in breast cancer cells. Carcinogenesis 25:1587–1592
Walker JE, Chen RX, Mcgee J, Nacey C, Pollard RB, Abedi M, Bauer G, Nolta JA, Anderson JS (2012) Generation of an HIV-1-resistant immune system with CD34+ hematopoietic stem cells transduced with a triple-combination anti-HIV lentiviral vector. J Virol 86:5719–5729
Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R (2013) One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153:910–918
Woong YH, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Joanna Yeh JR, Joung JK (2013) Efficient in vivo genome editing using RNA-guided nucleases. Nat Biotechnol 31:227–229
Wu QW, She HQ, Liang J, Huang YF, Yang QM, Yang QL, Zhang ZM (2012) Expression and clinical significance of extracellular matrix protein 1 and vascular endothelial growth factor-C in lymphatic metastasis of human breast cancer. BMC Cancer 12:47–58
Wu Q, Li X, Yang H, Lu C, You J, Zhang Z (2014) Extracellular matrix protein 1 is correlated to carcinogenesis and lymphatic metastasis of human gastric cancer. World J Surg Oncol 12:132–139
Xiao D, Zhang W, Li Y, Liu K, Zhao J, Sun X, Shan L, Mao Q, Xia H (2016) A novel luciferase knock-in reporter system for studying transcriptional regulation of the human Sox2 gene. J Biotechnol 219:110–116
Xu K, Zhang Y, Ilalov K, Carlson CS, Feng JQ, Di Cesare PE, Liu C (2007) Cartilage oligomeric matrix protein associates with granulin-epithelin precursor (GEP) and potentiates GEP-stimulated chondrocyte proliferation. J Biol Chem 282:11347–11355
Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, Jaenisch R (2013) One-step generation of mice carrying reporter and conditional alleles by CRISPR/Casmediated genome engineering. Cell 154:1370–1379
Acknowledgements
This study was supported by the research grants to H.X. from the National Natural Science Foundation of China (No. 81471772 and No. 81773265) and Key research and development plan of Shaanxi Province (No.2018SF-106).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declares that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Y., Zhang, W., Zhao, J. et al. Establishing a dual knock-out cell line by lentivirus based combined CRISPR/Cas9 and Loxp/Cre system. Cytotechnology 70, 1595–1605 (2018). https://doi.org/10.1007/s10616-018-0252-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-018-0252-2