Abstract
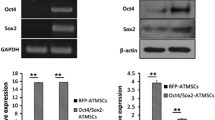
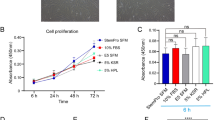
The proliferation and migration of mesenchymal stem cells (MSCs) are the efficiency determinants in MSCs transplant therapy. Sertoli cells considered as “nurse cell” possesses the ability to enhance the proliferation and migration of umbilical cord mesenchymal stem cells (UCMSCs). However, no reports about TM4 cells' effect on the proliferation and migration of adipose tissue-derived mesenchymal stem cells (ADSCs) have been found until at present research work. Therefore, this study investigates the effect of TM4 cells on the proliferation and migration of ADSCs. We found that the performance of proliferation and migration of ADSCs were improved significantly while maintaining their stemness and reducing their apoptosis rate. After co-culturing with TM4 cells, the co-cultured ADSCs demonstrated higher proportion of synthetic phase (S) cells and colony-forming units-fibroblastic (CFU-F) number, lower proportion of sub-G1 phase cells and enhanced osteogenic and adipogenic differentiation ability. Moreover, results confirmed the higher multiple proteins involved in cell proliferation and migration including expression of the phospho-Akt, mdm2, pho-CDC2, cyclin D1 CXCR4, MMP-2, as well as phospho-p44 MAPK and phospho-p38 MAPK in co-cultured ADSCs. Furthermore, the process of TM4 cells promoting the proliferation of ADSCs was significantly inhibited by the administration of the PI3K/AKT inhibitor LY294002. Obtained results indicated that TM4 cells through MAPK/ERK1/2, MAPK/p-38 and PI3K/Akt pathways influence the proliferation and migration of ADSCs. These findings indicated that TM4 cells were found effective in promoting stemness and migration of ADSCs, that proves adopted co-culturing technique as an efficient approach to obtain ADSCs in transplantation therapy.







Similar content being viewed by others
References
Baer PC, Schubert R, Bereiter-Hahn J, Plosser M, Geiger H (2009) Expression of a functional epidermal growth factor receptor on human adipose-derived mesenchymal stem cells and its signaling mechanism. Eur J Cell Biol 885:273–283
Baer PC, Griesche N, Luttmann W, Schubert R, Luttmann A, Geiger H (2010) Human adipose-derived mesenchymal stem cells in vitro: evaluation of an optimal expansion medium preserving stemness. Cytotherapy 121:96–106
Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R (2000) Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: implications for their use in cell therapy. Exp Hematol 286:707–715
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 966:857–868
Cai A, Qiu R, Li L, Zheng D, Dong Y, Yu D, Huang Y, Rao S, Zhou Y, Mai W (2013) Atorvastatin treatment of rats with ischemia-reperfusion injury improves adipose-derived mesenchymal stem cell migration and survival via the SDF-1alpha/CXCR-4 axis. PLoS ONE 812:e79100
Cho HH, Kyoung KM, Seo MJ, Kim YJ, Bae YC, Jung JS (2006) Overexpression of CXCR4 increases migration and proliferation of human adipose tissue stromal cells. Stem Cells Dev 156:853–864
Choi SC, Kim SJ, Choi JH, Park CY, Shim WJ, Lim DS (2008) Fibroblast growth factor-2 and -4 promote the proliferation of bone marrow mesenchymal stem cells by the activation of the PI3 K-Akt and ERK1/2 signaling pathways. Stem Cells Dev 174:725–736
Fan P, He L, Pu D, Lv XH, Zhou WX, Sun YN, Hu N (2011) Testicular sertoli cells influence the proliferation and immunogenicity of co-cultured endothelial cells. Biochem Biophys Res Commun 4043:829–833
Fenxi Z, Yan H, Wenmei L, Tongming R, Suhua J, Juntang L (2012) Co-culture with Sertoli cells promotes proliferation and migration of umbilical cord mesenchymal stem cells. Biochem Biophys Res Commun 4271:86–90
Garcia-Olmo D, Herreros D, Pascual M, Pascual I, De-La-Quintana P, Trebol J, Garcia-Arranz M (2009) Treatment of enterocutaneous fistula in Crohn’s Disease with adipose-derived stem cells: a comparison of protocols with and without cell expansion. Int J Colorectal Dis 241:27–30
Guo J, Jie W, Shen Z, Li M, Lan Y, Kong Y, Guo S, Li T, Zheng S (2014) SCF increases cardiac stem cell migration through PI3 K/AKT and MMP2/9 signaling. Int J Mol Med 341:112–118
Halvorsen YC, Wilkison WO, Gimble JM (2000) Adipose-derived stromal cells-their utility and potential in bone formation. Int J Obes 24(Suppl 4):S41–S44
Halvorsen YD, Franklin D, Bond AL, Hitt DC, Auchter C, Boskey AL, Paschalis EP, Wilkison WO, Gimble JM (2001) Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng 76:729–741
Huleihel M, Lunenfeld E (2004) Regulation of spermatogenesis by paracrine/autocrine testicular factors. Asian J Androl 63:259–268
Kim YS, Kwon JS, Hong MH, Kim J, Song CH, Jeong MH, Cho JG, Park JC, Kang JC, Ahn Y (2010) Promigratory activity of oxytocin on umbilical cord blood-derived mesenchymal stem cells. Artif Organs 346:453–461
Konno M, Hamazaki TS, Fukuda S, Tokuhara M, Uchiyama H, Okazawa H, Okochi H, Asashima M (2010) Efficiently differentiating vascular endothelial cells from adipose tissue-derived mesenchymal stem cells in serum-free culture. Biochem Biophys Res Commun 4004:461–465
Konno M, Hamabe A, Hasegawa S, Ogawa H, Fukusumi T, Nishikawa S, Ohta K, Kano Y, Ozaki M, Noguchi Y et al (2013) Adipose-derived mesenchymal stem cells and regenerative medicine. Dev Growth Differ 553:309–318
Liao X, Li F, Wang X, Yanoso J, Niyibizi C (2008) Distribution of murine adipose-derived mesenchymal stem cells in vivo following transplantation in developing mice. Stem Cells Dev 172:303–314
Lin G, Wang G, Liu G, Yang LJ, Chang LJ, Lue TF, Lin CS (2009) Treatment of type 1 diabetes with adipose tissue-derived stem cells expressing pancreatic duodenal homeobox 1. Stem Cells Dev 1810:1399–1406
Lu T, Xiong H, Wang K, Wang S, Ma Y, Guan W (2014) Isolation and characterization of adipose-derived mesenchymal stem cells (ADSCs) from cattle. Appl Biochem Biotechnol 1742:719–728
Majumdar MK, Banks V, Peluso DP, Morris EA (2000) Isolation, characterization, and chondrogenic potential of human bone marrow-derived multipotential stromal cells. J Cell Physiol 1851:98–106
Mather JP (1980) Establishment and characterization of two distinct mouse testicular epithelial cell lines. Biol Reprod 231:243–252
Mather JP, Zhuang LZ, Perez-Infante V, Phillips DM (1982) Culture of testicular cells in hormone-supplemented serum-free medium. Ann N Y Acad Sci 383:44–68
Mesimaki K, Lindroos B, Tornwall J, Mauno J, Lindqvist C, Kontio R, Miettinen S, Suuronen R (2009) Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg 383:201–209
Miryounesi M, Nayernia K, Dianatpour M, Mansouri F, Modarressi MH (2013) Co-culture of mouse embryonic stem cells with sertoli cells promote in vitro generation of germ cells. Iran J Basic Med Sci 166:779–783
Monfared MH, Minaee B, Rastegar T, Khrazinejad E, Barbarestani M (2016) Sertoli cell condition medium can induce germ like cells from bone marrow derived mesenchymal stem cells. Iran J Basic Med Sci 1911:1186–1192
Okura H, Komoda H, Fumimoto Y, Lee CM, Nishida T, Sawa Y, Matsuyama A (2009) Transdifferentiation of human adipose tissue-derived stromal cells into insulin-producing clusters. J Artif Organs 122:123–130
Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M et al (2004) Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 1095:656–663
Rangappa S, Fen C, Lee EH, Bongso A, Sim EK (2003) Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg 753:775–779
Roberts KP (2005) Chapter 19—sertoli cell lines. In: Sertoli cell biology. Academic Press, San Diego, pp 329–342
Ryu CH, Park SA, Kim SM, Lim JY, Jeong CH, Jun J, Oh JH, Park SH, Oh W-I, Jeun SS (2010) Migration of human umbilical cord blood mesenchymal stem cells mediated by stromal cell-derived factor-1/CXCR4 axis via Akt, ERK, and p38 signal transduction pathways. Biochem Biophys Res Commun 3981:105–110
Shi M, Li J, Liao L, Chen B, Li B, Chen L, Jia H, Zhao RC (2007) Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica 927:897–904
Simon L, Ekman GC, Tyagi G, Hess RA, Murphy KM, Cooke PS (2007) Common and distinct factors regulate expression of mRNA for ETV5 and GDNF, sertoli cell proteins essential for spermatogonial stem cell maintenance. Exp Cell Res 31314:3090–3099
Skinner MK (2005) Chapter 8—sertoli cell secreted regulatory factors. In: Sertoli cell biology. Academic Press, San Diego, pp 107–120
Tian H, Guo MJ, Zhuang YP, Chu J, Zhang SL (2014) Enhanced proliferation of bone marrow mesenchymal stem cells by co-culture with TM4 mouse Sertoli cells: involvement of the EGF/PI3 K/AKT pathway. Mol Cell Biochem 3931–2:155–164
Tobita M, Tajima S, Mizuno H (2015) Adipose tissue-derived mesenchymal stem cells and platelet-rich plasma: stem cell transplantation methods that enhance stemness. Stem Cell Res Ther 6:215
Wang J, Qu TB, Chu LS, Li L, Ren CC, Sun SQ, Fang Y (2016) Ligustrazine promoted the migration of bone marrow mesenchymal stem cells by Up-regulating MMP-2 and MMP-9 expressions. Chin J Integr Tradit West Med 366:718–723
Wei Y, Fang J, Cai S, Lv C, Zhang S, Hua J (2016) Primordial germ cell-like cells derived from canine adipose mesenchymal stem cells. Cell Prolif 494:503–511
Wynn RF, Hart CA, Corradi-Perini C, O’neill L, Evans CA, Wraith JE, Fairbairn LJ, Bellantuono I (2004) A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 1049:2643–2645
Yamamoto T, Gotoh M, Hattori R, Toriyama K, Kamei Y, Iwaguro H, Matsukawa Y, Funahashi Y (2010) Periurethral injection of autologous adipose-derived stem cells for the treatment of stress urinary incontinence in patients undergoing radical prostatectomy: report of two initial cases. Int J Urol 171:75–82
Yan Y, Ma T, Gong K, Ao Q, Zhang X, Gong Y (2014) Adipose-derived mesenchymal stem cell transplantation promotes adult neurogenesis in the brains of Alzheimer’s disease mice. Neural Regen Res 98:798–805
Yanez R, Lamana ML, Garcia-Castro J, Colmenero I, Ramirez M, Bueren JA (2006) Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells (Dayton, Ohio) 2411:2582–2591
Yin L, Zhu Y, Yang J, Ni Y, Zhou Z, Chen Y, Wen L (2015) Adipose tissue-derived mesenchymal stem cells differentiated into hepatocyte-like cells in vivo and in vitro. Mol Med Rep 113:1722–1732
Yue F, Cui L, Johkura K, Ogiwara N, Sasaki K (2006) Induction of midbrain dopaminergic neurons from primate embryonic stem cells by coculture with sertoli cells. Stem Cells (Dayton, Ohio) 247:1695–1706
Zhang F, Hong Y, Liang W, Ren T, Jing S, Lin J (2012) Co-culture withSertoli cells promotes proliferation and migration of umbilical cord mesenchymal stem cells. Biochem Biophys Res Commun 427(1):86–90
Zhang Z, Li S, Cui M, Gao X, Sun D, Qin X, Narsinh K, Li C, Jia H, Li C et al (2013) Rosuvastatin enhances the therapeutic efficacy of adipose-derived mesenchymal stem cells for myocardial infarction via PI3 K/Akt and MEK/ERK pathways. Basic Res Cardiol 1082:333
Zhang F, Lu M, Liu H, Ren T, Miao Y, Wang J (2016) Sertoli cells promote proliferation of bone marrow-derived mesenchymal stem cells in co-culture. Indian J Exp Biol 545:309–314
Zhang L, Xu P, Wang X, Zhang M, Yan Y, Chen Y, Zhang L, Zhang L (2017) Activin B regulates adipose-derived mesenchymal stem cells to promote skin wound healing via activation of the MAPK signaling pathway. Int J Biochem Cell Biol 87:69–76
Zhou H, Yang J, Xin T, Li D, Guo J, Hu S, Zhou S, Zhang T, Zhang Y, Han T et al (2014) Exendin-4 protects adipose-derived mesenchymal stem cells from apoptosis induced by hydrogen peroxide through the PI3 K/Akt-Sfrp2 pathways. Free Radic Biol Med 77:363–375
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 72:211–228
Funding
This research was supported by grants from the National Natural Science Foundation of China (81373286), National Basic Research Program of China (2011CBA00800), and the Fundamental Research Funds for the China Central Universities (Nos. 22221818014 and 22221817014).
Author information
Authors and Affiliations
Contributions
This study was designed by MG and HH and experiments were performed by YL, XC and QW. The experimental data was also analyzed by YL. The paper was written by AM, YL and MG. JC and YZ participated in the data discussion. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Availability of data and materials
All supporting data are included within the article and its Additional file.
Consent for publication
All authors consented to publication of the present manuscript.
Ethics approval and consent to participate
The authors declare that this is not a study involving human participants and reporting health related outcomes.
Rights and permissions
About this article
Cite this article
Luo, Y., Mohsin, A., Xu, C. et al. Co-culture with TM4 cells enhances the proliferation and migration of rat adipose-derived mesenchymal stem cells with high stemness. Cytotechnology 70, 1409–1422 (2018). https://doi.org/10.1007/s10616-018-0235-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-018-0235-3