Abstract
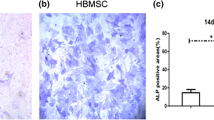
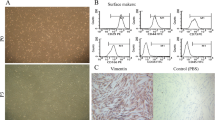
So far, substantial attentions have been attracted to the application of mesenchymal stem or stromal cells (MSCs) in different therapeutic approaches. Although human bone marrow is commonly considered as a major source for MSCs, having an invasive collection method, ethical consideration and donor availability create a challenge for scientists, leading them to explore better alternative sources for MSCs. The study presented here aimed to characterize and compare osteogenic capacity of MSCs obtained from the amnion membrane (AM) with those originated from BM. Cells isolated from AMs and BMs were cultured in DMEM-low glucose supplemented with FBS, penicillin and streptomycin. After 24 h of incubation, cells adhered to the plastic surface of the flasks were allowed to proliferate for more days. A sub-confluent culture of cells was trypsinized and re-cultured. The MSCs were characterized by the expression of specific markers with flow cytometry. The osteogenic differentiation of MSCs was also validated by alkaline phosphatase and alizarian red S staining. Our results showed comparable expression of MSCs specific markers for both MSC sources (AM and BM). We also showed the optimum osteogenic differentiation of MSCs from both sources whereas hAM-MSCs revealed higher proliferation rate. We found no essential immunophenotypic differences between MSCs originated from bone marrow and amnion membrane while their differentiations into osteoblastic linage were also comparable. This was in addition to the higher proliferation rate observed for hAM-MSCs which suggests hAM as an easily accessible and reliable source of MSCs applicable for bone engineering, regenerative medicine or other therapeutic approaches.



Similar content being viewed by others
References
Ahmadi M, Hosseini E, et al (2015) The role of amnion membrane-derived mesenchymal stem cells on differentiation and expansion of natural killer cell progenitors originated from umbilical cord blood mononuclear cells. Biotechnol Health Sci 2:36–39
Barry FP (2003) Mesenchymal stem cell therapy in joint disease. Novartis Found Symp 249:86–96 (discussion 96–102, 170–104, 239–141)
Bernardo ME, Locatelli F (2016) Mesenchymal stromal cells in hematopoietic stem cell transplantation. Methods Mol Biol 1416:3–20
Caplan AI (2007) Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213:341–347
Caplan AI (2009) Why are MSCs therapeutic? New data: new insight. J Pathol 217:318–324
Celebi B, Mantovani D et al (2011) Irradiated mesenchymal stem cells improve the ex vivo expansion of hematopoietic progenitors by partly mimicking the bone marrow endosteal environment. J Immunol Methods 370:93–103
Chen WM, Chen ZX et al (2008) Osteoblasts from patients with myelodysplastic syndrome express multiple cytokines and support hematopoietic progenitor cell survival in vitro. Zhongguo Shi Yan Xue Ye Xue Za Zhi 16:78–83
Conrad C, Zeindl-Eberhart E et al (2008) Alkaline phosphatase, glutathione-S-transferase-P, and cofilin-1 distinguish multipotent mesenchymal stromal cell lines derived from the bone marrow versus peripheral blood. Stem Cells Dev 17:23–27
de Barros AP, Takiya CM et al (2010) Osteoblasts and bone marrow mesenchymal stromal cells control hematopoietic stem cell migration and proliferation in 3D in vitro model. PLoS ONE 5:e9093
Deans RJ, Moseley AB (2000) Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol 28:875–884
Ding DC, Chang YH et al (2015) Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant 24:339–347
Du X, Yuan Q et al (2016) Endometrial mesenchymal stem cells isolated from menstrual blood by adherence. Stem Cells Int 2016:3573846
Fajardo-Orduna GR, Mayani H et al (2015) Hematopoietic support capacity of mesenchymal stem cells: biology and clinical potential. Arch Med Res 46:589–596
Fajardo-Orduna G, Mayani H et al (2016) Bone marrow mesenchymal stromal cells from clinical scale culture: in vitro evaluation of their differentiation, hematopoietic support and immunosuppressive capacities. Stem Cells Dev 25:1299–1310
Fujita S, Toguchida J et al (2008) Clonal analysis of hematopoiesis-supporting activity of human mesenchymal stem cells in association with Jagged1 expression and osteogenic potential. Cell Transpl 17:1169–1179
He Q, Wan C et al (2007) Concise review: multipotent mesenchymal stromal cells in blood. Stem cells (Dayton, Ohio) 25:69–77
Herzog EL, Chai L et al (2003) Plasticity of marrow-derived stem cells. Blood 102:3483–3493
Honmou O, Onodera R et al (2012) Mesenchymal stem cells: therapeutic outlook for stroke. Trends Mol Med 18:292–297
Hoogduijn MJ, Crop MJ et al (2007) Human heart, spleen, and perirenal fat-derived mesenchymal stem cells have immunomodulatory capacities. Stem Cells Dev 16:597–604
Hosseini E, Ghasemzadeh M et al (2017) Ex vivo expansion of CD3 depleted cord blood-MNCs in the presence of bone marrow stromal cells; an appropriate strategy to provide functional NK cells applicable for cellular therapy. Stem Cell Res 19:148–155
Huang XB, Liu T et al (2006) Osteoblasts differentiated from human marrow bone mesenchymal stem cells support hematopoietic stem/progenitor cells from umbilical cord blood. Zhongguo Shi Yan Xue Ye Xue Za Zhi 14:552–556
Hung SC, Chen NJ et al (2002) Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells 20:249–258
In’t Anker PS, Scherjon SA et al (2004) Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 22:1338–1345
Jackson WM, Nesti LJ et al (2012) Concise review: clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl Med 1:44–50
Jin HJ, Bae YK et al (2013) Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci 14:17986–18001
Jo Y, Lee H et al (2007) Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng 13:767–773
Jung Y, Wang J et al (2005) Cell-to-cell contact is critical for the survival of hematopoietic progenitor cells on osteoblasts. Cytokine 32:155–162
Kilic E, Ceyhan T et al (2007) Evaluation of differentiation potential of human bone marrow-derived mesenchymal stromal cells to cartilage and bone cells. Acta Orthop Traumatol Turc 41:295–301
Klein C, Strobel J et al (2013) Ex vivo expansion of hematopoietic stem- and progenitor cells from cord blood in coculture with mesenchymal stroma cells from amnion, chorion, Wharton’s jelly, amniotic fluid, cord blood, and bone marrow. Tissue Eng Part A 19:2577–2585
Kuo Ching Chao KFC (2008) Islet-like clusters derived from mesenchymal stem cells in Wharton’s jelly of the human umbilical cord for transplantation to control type 1 diabetes. Public Library of Science
Li Q, Zhou X et al (2013) In vivo tracking and comparison of the therapeutic effects of MSCs and HSCs for liver injury. PLoS ONE 8:e62363
Lindenmair A, Hatlapatka T et al (2012) Mesenchymal stem or stromal cells from amnion and umbilical cord tissue and their potential for clinical applications. Cells 1:1061–1088
Madrigal M, Rao K et al (2014) A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med 12:260
Mafi P, Hindocha S et al (2011) Adult mesenchymal stem cells and cell surface characterization: a systematic review of the literature. Open Orthop J 5:253–260
Manochantr S, Tantrawatpan C et al (2010) Isolation, characterization and neural differentiation potential of amnion derived mesenchymal stem cells. J Med Assoc Thai 93:S183–S191
Miura Y (2013) Regulation of hematopoiesis by mesenchymal stem cells. Rinsho Ketsueki 54:431–435
Mobasheri A, Csaki C et al (2009) Mesenchymal stem cells in connective tissue engineering and regenerative medicine: applications in cartilage repair and osteoarthritis therapy. Histol Histopathol 24:347–366
Moon JH, Lee JR et al (2008) Successful vitrification of human amnion-derived mesenchymal stem cells. Hum Reprod 23:1760–1770
Neve A, Corrado A et al (2011) Osteoblast physiology in normal and pathological conditions. Cell Tissue Res 343:289–302
Nuschke A (2014) Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis 10:29–37
Ochsenbein-Kolble N, Bilic G et al (2003) Inducing proliferation of human amnion epithelial and mesenchymal cells for prospective engineering of membrane repair. J Perinat Med 31:287–294
Ogura N, Kawada M et al (2004) Differentiation of the human mesenchymal stem cells derived from bone marrow and enhancement of cell attachment by fibronectin. J Oral Sci 46:207–213
Oh W, Kim DS et al (2008) Immunological properties of umbilical cord blood-derived mesenchymal stromal cells. Cell Immunol 251:116–123
Pittenger MF, Mackay AM et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Pontikoglou C, Deschaseaux F et al (2011) Bone marrow mesenchymal stem cells: biological properties and their role in hematopoiesis and hematopoietic stem cell transplantation. Stem Cell Rev 7:569–589
Portmann-Lanz CB, Schoeberlein A et al (2006) Placental mesenchymal stem cells as potential autologous graft for pre-and perinatal neuroregeneration. Am J Obstet Gynecol 194:664–673
Post S, Abdallah BM et al (2008) Demonstration of the presence of independent pre-osteoblastic and pre-adipocytic cell populations in bone marrow-derived mesenchymal stem cells. Bone 43:32–39
Ramakrishnan A, Torok-Storb B et al (2013) Primary marrow-derived stromal cells: isolation and manipulation. Methods Mol Biol 1035:75–101
Ren H, Sang Y et al (2016) Comparative analysis of human mesenchymal stem cells from umbilical cord, dental pulp, and menstrual blood as sources for cell therapy. Stem Cells Int 2016:3516574
Schmal O, Seifert J et al (2016) Hematopoietic stem and progenitor cell expansion in contact with mesenchymal stromal cells in a hanging drop model uncovers disadvantages of 3D culture. Stem Cells Int 2016:4148093
Sensebe L, Bourin P (2009) Mesenchymal stem cells for therapeutic purposes. Transplantation 87:S49–S53
Shen JL, Huang YZ et al (2012) Effectiveness of human mesenchymal stem cells derived from bone marrow cryopreserved for 23–25 years. Cryobiology 64:167–175
Shiozawa Y, Takenouchi H et al (2008) Human osteoblasts support hematopoietic cell development in vitro. Acta Haematol 120:134–145
Shu J, Guo L et al (2011) Experimental study on the induced differentiation of human amnion mesenchymal cells into osteoblasts. Zhonghua zheng xing wai ke za zhi = Zhonghua zhengxing waike zazhi = Chin J Plast Surg 27:362–367
Tae SK, Lee SH et al (2006) Mesenchymal stem cells for tissue engineering and regenerative medicine. Biomed Mater 1:63–71
Verfaillie CM (1998) Adhesion receptors as regulators of the hematopoietic process. Blood 92:2609–2612
Walenda T, Bokermann G et al (2011) Synergistic effects of growth factors and mesenchymal stromal cells for expansion of hematopoietic stem and progenitor cells. Exp Hematol 39:617–628
Wang L, Li ZY et al (2015a) Dynamic expression profiles of marker genes in osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Chin Med Sci J 30:108–113
Wang Y, Yin Y et al (2015b) Human amnion mesenchymal stem cells promote proliferation and osteogenic differentiation in human bone marrow mesenchymal stem cells. J Mol Histol 46:13–20
Wang Y, Jiang F et al (2016a) Human amnion-derived mesenchymal stem cells promote osteogenic differentiation in human bone marrow mesenchymal stem cells by influencing the ERK1/2 signaling pathway. Stem Cells Int 2016:4851081
Wang Y, Yu X et al (2016b) Liver-derived human mesenchymal stem cells: a novel therapeutic source for liver diseases. Stem Cell Res Ther 7:71
Wei X, Yang X et al (2013) Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin 34:747–754
Yang ZX, Han ZB et al (2013) CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS ONE 8:e59354
Zannettino AC, Paton S et al (2008) Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol 214:413–421
Zhao K, Liu Q (2016) The clinical application of mesenchymal stromal cells in hematopoietic stem cell transplantation. J Hematol Oncol 9:46
Acknowledgements
This work was part of Dr. Hosseini’s approved project (No. 1390-01-33-1516) supported by Iranian blood transfusion organization and High Institute for Research and Education in Transfusion Medicine in Iran. The authors declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghasemzadeh, M., Hosseini, E., Ahmadi, M. et al. Comparable osteogenic capacity of mesenchymal stem or stromal cells derived from human amnion membrane and bone marrow. Cytotechnology 70, 729–739 (2018). https://doi.org/10.1007/s10616-017-0177-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-017-0177-1