Abstract
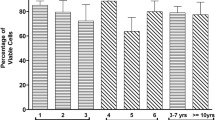
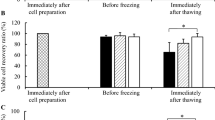
Off-the-shelf availability of human adipose-derived mesenchymal stromal cells (ASCs) for regenerative medicine application requires the development of nontoxic, safe, and efficient protocols for cryopreservation. Favorably, such cell processing protocols should not contain xenogeneic or toxic components, such as fetal bovine serum (FS) and dimethyl sulfoxide (DMSO). The objective of the study was to assess the sensitivity of ASCs to DMSO-free cryopreservation protocol depending on their expansion conditions: conventional, based on the application of FS or xeno-free, using PL as a medium supplement. ASCs expansion was carried out in α-MEM supplemented either with FS or PL. For DMSO- and xeno-free cryopreservation ASCs were pretreated with different concentrations of sucrose during 24 h of culture. Pretreated ASCs were cryopreserved in α-MEM containing 100–300 mM of sucrose with the cooling rate of 1 degree/min. ASCs were tested for survival (Trypan Blue test), viability (MTT test), recovery (Alamar Blue test), proliferation and ability to multilineage differentiation. The optimal concentrations of sucrose for ASCs pretreatment and as an additive in cryoprotective solution, which provided highest cell survival, comprised 100 and 200 mM, correspondingly. Survival and recovery rates of platelet lysate (PL)-expanded ASCs after DMSO-free cryopreservation comprised 59 and 51%, and were higher than in FS-cultured cells. After DMSO-free cryopreservation PL-processed ASCs had a shorter population doubling time and higher capacity for osteogenic differentiation than FS-processed cultures. The described DMSO- and xeno-free processing may form the basis for the development of safe and efficient protocols for manufacturing and banking of ASCs, providing their off-the-shelf availability for regenerative medicine applications.




Similar content being viewed by others
References
Astori G, Amati E, Bambi F, Bernardi M, Chieregato K, Schäfer R, Sella S, Rodeghiero F (2016) Platelet lysate as a substitute for animal serum for the ex vivo expansion of mesenchymal stem/stromal cells: present and future. Stem Cell Res Ther 7:93. doi:10.1186/s13287-016-0352-x
Barry FP, Murphy JM (2004) Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol 36:568–584. doi:10.1016/j.biocel.2003.11.001
Bernardo ME, Avanzini MA, Perotti C, Cometa AM, Moretta A, Lenta E, Del Fante C, Novara F, de Silvestri A, Amendola G, Zuffardi O, Maccario R, Locatelli F (2007) Optimization of in vitro expansion of human multipotent mesenchymal stromal cells for cell-therapy approaches: further insights in the search for a fetal calf serum substitute. J Cell Physiol 211:121–130. doi:10.1002/jcp.20911
Blande IS, Bassaneze V, Lavini-Ramos C, Fae KC, Kalil J, Miyakawa AA, Schettert IT, Krieger JE (2009) Adipose tissue mesenchymal stem cell expansion in animal serum-free medium supplemented with autologous human platelet lysate. Transfusion 49:2680–2685. doi:10.1111/j.1537-2995.2009.02346.x
Blasi A, Martino C, Balducci L, Saldarelli M, Soleti A, Navone SE, Canzi L, Cristini S, Invernici G, Parati EA, Alessandri G (2011) Dermal fibroblasts display similar phenotypic and differentiation capacity to fat-derived mesenchymal stem cells, but differ in anti-inflammatory and angiogenic potential. Vasc Cell 3:5. doi:10.1186/2045-824X-3-5
Buchanan SS, Gross SA, Acker JP, Toner M, Carpenter JF, Pyatt DW (2004) Cryopreservation of stem cells using trehalose: evaluation of the method using a human hematopoietic cell line. Stem Cells Dev 13:295–305. doi:10.1089/154732804323099226
Campbell LH, Brockbank KG (2012) Culturing with trehalose produces viable endothelial cells after cryopreservation. Cryobiology 64:240–244. doi:10.1016/j.cryobiol.2012.02.006
Chevallier N, Anagnostou F, Zilber S, Bodivit G, Maurin S, Barrault A, Bierling P, Hernigou P, Layrolle P, Rouard H (2010) Osteoblastic differentiation of human mesenchymal stem cells with platelet lysate. Biomaterials 31:270–278. doi:10.1016/j.biomaterials.2009.09.043
Davies OG, Smith AJ, Cooper PR, Shelton RM, Scheven BA (2014) The effects of cryopreservation on cells isolated from adipose, bone marrow and dental pulp tissues. Cryobiology 69:342–347. doi:10.1016/j.cryobiol.2014.08.003
Doucet C, Ernou I, Zhang Y, Llense JR, Begot L, Holy X, Lataillade JJ (2005) Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol 205:228–236. doi:10.1002/jcp.20391
Dumont F, Marechal PA, Gervais P (2004) Cell size and water permeability as determining factors for cell viability after freezing at different cooling rates. Appl Environ Microbiol 70:268–272. doi:10.1128/AEM.70.1.268-272.2004
Gimble JM, Katz AJ, Bunnell BA (2007) Adipose-derived stem cells for regenerative medicine. Circ Res 100:1249–1260. doi:10.1161/01.RES.0000265074.83288.09
Goh BC, Thirumala S, Kilroy G, Devireddy RV, Gimble JM (2007) Cryopreservation characteristics of adipose-derived stem cells: maintenance of differentiation potential and viability. J Tissue Eng Regen Med 1:322–324. doi:10.1002/term.35
Gottipamula S, Sharma A, Krishnamurthy S, Majumdar AS, Seetharam RN (2012) Human platelet lysate is an alternative to fetal bovine serum for large-scale expansion of bone marrow-derived mesenchymal stromal cells. Biotechnol Lett 34:1367–1374. doi:10.1007/s10529-012-0893-8
Griffiths S, Baraniak PR, Copland IB, Nerem RM, McDevitt TC (2013) Human platelet lysate stimulates high-passage and senescent human multipotent mesenchymal stromal cell growth and rejuvenation in vitro. Cytotherapy 15:1469–1483. doi:10.1016/j.jcyt.2013.05.020
Gruber R, Karreth F, Kandler B, Fuerst G, Rot A, Fischer M, Watzek G (2004) Platelet-released supernatants increase migration and proliferation, and decrease osteogenic differentiation of bone marrow-derived mesenchymal progenitor cells under in vitro conditions. Platelets 15:29–35. doi:10.1080/09537100310001643999
Haasters F, Prall WC, Anz D, Bourquin C, Pautke C, Endres S, Mutschler W, Docheva D, Schieker M (2009) Morphological and immunocytochemical characteristics indicate the yield of early progenitors and represent a quality control for human mesenchymal stem cell culturing. J Anat 214:759–767. doi:10.1111/j.1469-7580.2009.01065.x
Hammer Ø, Harper DAT, Ryan PD (2001) Paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9–18
Horn P, Bokermann G, Cholewa D, Bork S, Walenda T, Koch C, Drescher W, Hutschenreuther G, Zenke M, Ho AD, Wagner W (2010) Impact of individual platelet lysates on isolation and growth of human mesenchymal stromal cells. Cytotherapy 12:888–898. doi:10.3109/14653249.2010.501788
Jääger K, Islam S, Zajac P, Linnarsson S, Neuman T (2012) RNA-seq analysis reveals different dynamics of differentiation of human dermis- and adipose-derived stromal stem cells. PLoS ONE 7:e38833. doi:10.1371/journal.pone.0038833
Kinzebach S, Dietz L, Klüter H, Thierse H-J, Bieback K (2013) Functional and differential proteomic analyses to identify platelet derived factors affecting ex vivo expansion of mesenchymal stromal cells. BMC Cell Biol 14:48. doi:10.1186/1471-2121-14-48
Lampugnani MG, Pedenovi M, Niewiarowski A, Casali B, Donati MB, Corbascio GC, Marchisio PC (1987) Effects of dimethyl sulfoxide (DMSO) on microfilament organization, cellular adhesion, and growth of cultured mouse B16 melanoma cells. Exp Cell Res 172:385–396
Lange C, Cakiroglu F, Spiess AN, Cappallo-Obermann H, Dierlamm J, Zander AR (2007) Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J Cell Physiol 213:18–26. doi:10.1002/jcp.21081
Liu Y, Xu X, Ma XH, Liu J, Cui ZF (2011) Effect of various freezing solutions on cryopreservation of mesenchymal stem cells from different animal species. CryoLetters 32:425–435
Marquez-Curtis LA, Janowska-Wieczorek A, McGann LE, Elliott JAW (2015) Mesenchymal stromal cells derived from various tissues: biological, clinical and cryopreservation aspects. Cryobiology 71:181–197. doi:10.1016/j.cryobiol.2015.07.003
Matsumura K, Hayashi F, Nagashima T, Hyon SH (2013) Long-term cryopreservation of human mesenchymal stem cells using carboxylated poly-l-lysine without the addition of proteins or dimethyl sulfoxide. J Biomater Sci Polym Ed 24:1484–1497. doi:10.1080/09205063.2013.771318
Motta JP, Paraguassú-Braga FH, Bouzas LF, Porto LC (2014) Evaluation of intracellular and extracellular trehalose as a cryoprotectant of stem cells obtained from umbilical cord blood. Cryobiology 68:343–348. doi:10.1016/j.cryobiol.2014.04.007
Naaijkens BA, Niessen HWM, Prins H-J, Krijnen PA, Kokhuis TJ, de Jong N, van Hinsbergh VW, Kamp O, Helder MN, Musters RJ, van Dijk A, Juffermans LJ (2012) Human platelet lysate as a fetal bovine serum substitute improves human adipose-derived stromal cell culture for future cardiac repair applications. Cell Tissue Res 348:119–130. doi:10.1007/s00441-012-1360-5
Notman R, Noro M, O’Malley B, Anwar J (2006) Molecular basis for dimethylsulfoxide (DMSO) action on lipid membranes. J Am Chem Soc 128:13982–13983. doi:10.1021/ja063363t
Oliver AE, Jamil K, Crowe JH, Tablin F (2004) Loading human mesenchymal stem cells with trehalose by fluid-phase endocytosis. Cell Preserv Technol 2:35–49. doi:10.1089/153834404322708745
Petrenko YA, Petrenko AY (2012) Phenotypical properties and ability to multilineage differentiation of adipose tissue stromal cells during subculturing. Cytol Genet 46:36–40. doi:10.3103/S0095452712010070
Petrenko YA, Rogulska OY, Mutsenko VV, Petrenko AY (2014) A sugar pretreatment as a new approach to the DMSO- and xeno-free cryopreservation of human mesenchymal stromal cells. CryoLetters 35:239–246
Reboulleau CP, Shapiro HS (1983) Chemical inducers of differentiation cause conformational changes in the chromatin and deoxyribonucleic acid of murine erythroleukemia cells. Biochemistry 22:4512–4517
Reinisch A, Bartmann C, Rohde E, Schallmoser K, Bjelic-Radisic V, Lanzer G, Linkesch W, Strunk D (2007) Humanized system to propagate cord blood-derived multipotent mesenchymal stromal cells for clinical application. Regen Med 2:371–382. doi:10.2217/17460751.2.4.371
Rodrigues JP, Paraguassú-Braga FH, Carvalho L, Abdelhay E, Bouzas LF, Porto LC (2008) Evaluation of trehalose and sucrose as cryoprotectants for hematopoietic stem cells of umbilical cord blood. Cryobiology 56:144–151. doi:10.1016/j.cryobiol.2008.01.003
Rodriguez L, Velasco B, García J, Martín-Henao GA (2005) Evaluation of an automated cell processing device to reduce the dimethyl sulfoxide from hematopoietic grafts after thawing. Transfusion 45:1391–1397. doi:10.1111/j.1537-2995.2005.00213.x
Shivakumar SB, Bharti D, Jang SJ, Hwang SC, Park JK, Shin JK, Byun JH, Park BW, Rho GJ (2015) Cryopreservation of human Wharton’s Jelly-derived mesenchymal stem cells following controlled rate freezing protocol using different cryoprotectants; a comparative study. Int J Stem Cells 8:155–169. doi:10.15283/ijsc.2015.8.2.155
Skorobogatova NG, Novikov AN, Fuller BJ, Petrenko AY (2010) Importance of a three-stage cooling regime and induced ice nucleation during cryopreservation on colony-forming potential and differentiation in mesenchymal stem progenitor cells from human fetal liver. CryoLetters 31:371–379
Thirumala S, Goebel W, Woods E (2009) Clinical grade adult stem cell banking. Organogenesis 5:143–514
Thirumala S, Gimble JM, Devireddy RV (2010) Cryopreservation of stromal vascular fraction of adipose tissue in a serum-free freezing medium. J Tissue Eng Regen Med 4:224–232. doi:10.1002/term.232
Windrum P, Morris TCM, Drake MB, Niederwieser D, Ruutu T (2005) Variation in dimethyl sulfoxide use in stem cell transplantation: a survey of EBMT centres. Bone Marrow Transpl 36:601–603. doi:10.1038/sj.bmt.1705100
Woodbury D, Schwarz EJ, Prockop DJ, Black IB (2000) Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res 61:364–370. doi:10.1002/1097-4547(20000815)61:4<364:AID-JNR2>3.0.CO;2-C
Xia W, Li H, Wang Z, Xu R, Fu Y, Zhang X, Ye X, Huang Y, Xiang AP, Yu W (2011) Human platelet lysate supports ex vivo expansion and enhances osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Cell Biol Int 35:639–643. doi:10.1042/CBI20100361
Zaky SH, Ottonello A, Strada P, Cancedda R, Mastrogiacomo M (2008) Platelet lysate favours in vitro expansion of human bone marrow stromal cells for bone and cartilage engineering. J Tissue Eng Regen Med 2:472–481. doi:10.1002/term.119
Zuk P, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295. doi:10.1091/mbc.E02-02-0105
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rogulska, O., Petrenko, Y. & Petrenko, A. DMSO-free cryopreservation of adipose-derived mesenchymal stromal cells: expansion medium affects post-thaw survival. Cytotechnology 69, 265–276 (2017). https://doi.org/10.1007/s10616-016-0055-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-016-0055-2