Abstract
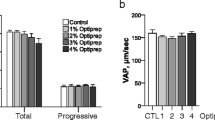
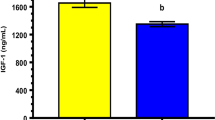
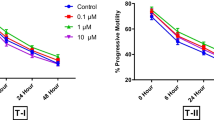
The objective of the study was to devise a cryoprotection synergism between glycerol and dimethyl sulfoxide (DMSO) for water buffalo spermatozoa. Additionally, the effect of best evolved concentrations of glycerol and DMSO in extender was assessed on in vivo fertility of buffalo spermatozoa. Ejaculates (n = 30) were equally distributed into five aliquots; first aliquot was diluted at 37 °C in extender having 7 % glycerol (control); second aliquot was diluted at 37 °C as well as at 4 °C in extender having 3.5 % DMSO (Group 1); third aliquot was diluted at 37 °C in extender having 3.5 % glycerol and then at 4 °C in extender having 3.5 % DMSO (Group 2); fourth aliquot was diluted at 37 °C in extender having 3.5 % DMSO and then at 4 °C in extender having 3.5 % glycerol (Group 3); fifth aliquot was diluted in extenders having 1.75 % glycerol and 1.75 % DMSO at 37 as well as at 4 °C (Group 4). At post thawing, sperm progressive motility (%), rapid velocity (%), average path velocity (µm/s), curved line velocity (µm/s), in vitro longevity (%), structural and functional integrity of plasmalemma (%), mitochondrial transmembrane potential (%) and viable sperm with intact acrosome (%) were higher (P < 0.05) in Group 4 compared to other treatment groups and control. Regarding sperm DNA integrity (%); it was higher (P < 0.05) in Group 4 compared to Group 1, 3 and control. The in vivo fertility (%) of buffalo spermatozoa was significantly higher with Group 4 compared to control (69.45 vs. 59.81). In conclusion, synergism exists between glycerol and DMSO (Group 4) in improving the quality and in vivo fertility of cryopreserved water buffalo spermatozoa.
Similar content being viewed by others
References
Ahmed H, Andrabi SMH, Jahan S (2016) Semen quality parameters as fertility predictors of water buffalo bull spermatozoa during low breeding season. Theriogenology 86:1516–1522
Aitken RJ, De Iuliis GN, Finnie JM, Hedges A, McLachlan RI (2010) Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Hum Reprod 25:2415–2426
Akhter S, Ansari MS, Andrabi SMH, Ullah N, Qayyum M (2008) Effect of antibiotics in extender on bacterial and spermatozoa quality of cooled buffalo (Bubalus bubalis) bull semen. Reprod Domest Anim 43:272–278
Andrabi SMH (2007) Mammalian sperm chromatin structure and assessment of DNA fragmentation. J Assist Reprod Genet 24:561–569
Andrabi SMH (2009) Factors affecting the quality of cryopreserved buffalo (Bubalus bubalis) bull spermatozoa. Reprod Domest Anim 44:552–569
Andrabi SMH (2014) Animal andrology: theories and applications. In: Chenoweth P, Lorton S (eds) Applied andrology in water buffalo. CABI, Wallingford, Oxfordshire, pp 380–403
Andrabi SMH, Ansari MS, Ullah N, Afzal M (2008a) Effect of non-enzymatic antioxidants in extender on post-thaw quality of buffalo (Bubalus bubalis) bull spermatozoa. Pak Vet J 28:159–162
Andrabi SMH, Ansari MS, Ullah N, Anwar M, Mehmood A, Akhter S (2008b) Duck egg yolk in extender improves the freezability of buffalo bull spermatozoa. Anim Reprod Sci 104:427–433
Anzar M, Kroetsch T, Boswall L (2011) Cryopreservation of bull semen shipped overnight and its effect on post-thaw sperm motility, plasma membrane integrity, mitochondrial membrane potential and normal acrosomes. Anim Reprod Sci 126:23–31
Arakawa T, Carpenter JF, Kita YA, Crowem JH (1990) The basis for toxicity of certain cryoprotectants: a hypothesis. Cryobiology 27:401–415
Bamba K, Adams CE (1990) Freezing rabbit semen by use of BF5 diluent. Lab Anim 24:172–175
Barratt CL, Björndahl L, Menkveld R, Mortimer D (2011) ESHRE special interest group for andrology basic semen analysis course: a continued focus on accuracy, quality, efficiency and clinical relevance. Hum Reprod 26:3207–3212
Blesbois E (2007) Current status in avian semen cryopreservation. World’s Poult Sci J 63:213–222
Brito LFC, Barth AD, Bilodeau-Goeseels S, Panich PL, Kastelic JP (2003) Comparison of methods to evaluate the plasmalemma of bovine sperm and their relationship with in vitro fertilization rate. Theriogenology 60:1539–1551
Buhr MM, Fiser P, Bailey JL, Curtis EF (2001) Cryopreservation in different concentrations of glycerol alters boar sperm and their membranes. J Androl 22:961–969
Büyükleblebici S, Tuncer PB, Taşdemir U, Özgürtaş T, Durmaz E, Büyükleblebici O (2014) The comparison of three different cryoprotectants in cryopreservation of angora goat semen. Kafkas Üniversitesi Veteriner Fakültesi Dergisi 20:613–619
Chan PJ, Tredway DR, Corselli J, Pang S, Su BC (1991) Combined supravital staining and hypoosmotic swelling. Hum Reprod 6:1115–1118
Dayem AMH, Mahmoud KGhM, Nawito MF, Ayoub MM, Darwish SF (2009) Genotyping of kappa-casein gene in Egyptian buffalo bulls. Livest Sci 122:286–289
Fiser PS, Fairfull RW (1989) The effect of glycerol-related osmotic changes on post-thaw motility and acrosomal integrity of ram spermatozoa. Cryobiology 26:64–69
Gallon F, Marchetti C, Jouy N, Marchetti P (2006) The functionality of mitochondria differentiates human spermatozoa with high and low fertilizing capability. Fertil Steril 86:1526–1530
Guthrie HD, Welch GR (2006) Determination of intracellular reactive oxygen species and high mitochondrial membrane potential in percoll-treated viable boar sperm using fluorescence-activated flow cytometry. J Anim Sci 84:2089–2100
Guthrie HD, Liu J, Critser JK (2002) Osmotic tolerance limits and effects of cryoprotectants on motility of bovine spermatozoa. Biol Reprod 67:1811–1816
Hammerstedt RH, Graham JK (1992) Cryopreservation of poultry sperm: the enigma of glycerol. Cryobiology 29:26–38
Hammerstedt RH, Graham JK, Nolan JP (1990) Cryopreservation of mammalian sperm: what we ask them to survive. J Androl 11:73–88
Holt WV (2000a) Fundamental aspects of sperm cryobiology: the importance of species and individual differences. Theriogenology 53:47–58
Holt WV (2000b) Basic aspects of frozen storage of semen. Anim Reprod Sci 62:3–22
Holt WV, O’Brien J, Abaigar T (2007) Applications and interpretation of computer-assisted sperm analyses and sperm sorting methods in assisted breeding and comparative research. Reprod Fertil Dev 19:709–718
Jeyendran RS, Van der Ven HH, Perez-Pelaez M, Zaneveld LJ (1985) Effect of glycerol and cryopreservation on oocyte penetration by human spermatozoa. Andrologia 17:241–248
Johnson MH, Nasr-Esfahani MH (1994) Radical solutions and cultural problems: could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? BioEssays 16:31–38
Kovács A, Foote RH (1992) Viability and acrosome staining of bull, boar and rabbit spermatozoa. Biotech Histochem 67:119–124
Long JA (2014) Animal andrology: theories and applications. In: Chenoweth P, Lorton S (eds) Applied andrology in chickens and turkeys. CABI, Wallingford, Oxfordshire, pp 197–225
Martins CF, Dode MN, Báo SN, Rumpf R (2007) The use of the acridine orange test and the TUNEL assay to assess the integrity of freeze-dried bovine spermatozoa DNA. Genet Mol Res 6:94–104
McLeskey SB, Dowds C, Carballada R, White RR, Saling PM (1998) Molecules involved in mammalian sperm-egg interaction. Int Rev Cytol 177:57–113
Medeiros CMO, Forell F, Oliveira ATD, Rodrigues JL (2002) Current status of sperm cryopreservation: why is it better. Theriogenology 57:327–344
Rasul Z, Ahmed N, Anzar M (2007) Antagonist effect of DMSO on the cryoprotection ability of glycerol during cryopreservation of buffalo sperm. Theriogenology 68:813–819
Shah SAH, Andrabi SMH, Qureshi IZ (2016) Effect of equilibration times, freezing and thawing rates on post-thaw quality of buffalo (Bubalus bubalis) bull spermatozoa. Andrology 4:972–976
Snedeker WH, Gaunya WS (1970) Dimethyl sulfoxide as a cryoprotective agent for freezing bovine semen. J Anim Sci 30:953–956
Sum AK, de Pablo JJ (2003) Molecular simulation study on the influence of dimethylsulfoxide on the structure of phospholipid bilayers. Biophys J 85:3636–3645
Wundrich K, Paasch U, Leicht M, Glander HJ (2006) Activation of caspases in human spermatozoa during cryopreservation—an immunoblot study. Cell Tissue Bank 7:81–90
Yu ZW, Quinn PJ (1994) Dimethyl sulfoxide: a review of its application in cell biology. Biosci Reports 14:259–281
Acknowledgments
The authors thank Pakistan Science Foundation (PSF), Islamabad for support under the Natural Sciences Linkages Programme (NSLP–213).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Shah, S.A.H., Andrabi, S.M.H., Ahmed, H. et al. Cryoprotection synergism between glycerol and dimethyl sulfoxide improves the mitochondrial transmembrane potential, plasmalemma, acrosomal and DNA integrities, and in vivo fertility of water buffalo (Bubalus bubalis) spermatozoa. Cytotechnology 68, 2335–2344 (2016). https://doi.org/10.1007/s10616-016-0027-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-016-0027-6