Abstract
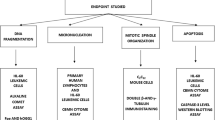
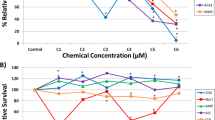
Micronucleus (MN) assay constitutes a valuable surrogate to the chromosome aberration technique for in vitro testing of the genotoxicity of substances. As test substances, two peptidic compounds (DOTATATE and Ubiquicidin29-41) used in nuclear medicine, were tested for in vitro cytotoxicity and genotoxicity in CHO-K1 cells. None of the compounds showed detectable cytotoxicity (0.5–7.3 ng/mL for DOTATATE and 0.3–4.5 ng/mL for UBI29-41), genotoxicity (0.72, 7.2 and 72.0 ng/ml for DOTATATE and 0.45, 4.5 and 45.0 ng/mL for UBI29-41) or cell cycle changes as compared to untreated controls at the concentrations tested. Statistical analysis showed good concordance between two independent analysts. The results corroborate the notion of the safety of the compounds and present improvements of the in vitro MN assay when performed in a pre-clinical trial context that increase the throughput of small-to-medium testing facilities as an alternative to high content screening systems.





Similar content being viewed by others
References
Akhtar MS, Qaisar A, Irfanullah J et al (2005) Antimicrobial Peptide 99mTc-Ubiquicidin29–41 as human infection-imaging agent: clinical trial. J Nucl Med 46:567–573
Bernardi M, Adami V, Albiero E et al (2014) Absence of micronucleus formation in CHO-K1 cells cultivated in platelet lysate enriched medium. Exp Toxicol Pathol 66:111–116. doi:10.1016/j.etp.2013.11.001
Bonassi S, El-Zein R, Bolognesi C, Fenech M (2011) Micronuclei frequency in peripheral blood lymphocytes and cancer risk: evidence from human studies. Mutagenesis 26:93–100. doi:10.1093/mutage/geq075
Casar B, Lopes C, Drljević A et al (2016) Medical physics in Europe following recommendations of the international atomic energy agency. Radiol Oncol 50:64–72. doi:10.1515/raon-2016-0004
Çavaş T (2008) In vivo genotoxicity of mercury chloride and lead acetate: micronucleus test on acridine orange stained fish cells. Food Chem Toxicol 46:352–358. doi:10.1016/j.fct.2007.08.015
Çelik A, Öǧenler O, Çömelekoǧlu Ü (2005) The evaluation of micronucleus frequency by acridine orange fluorescent staining in peripheral blood of rats treated with lead acetate. Mutagenesis 20:411–415. doi:10.1093/mutage/gei055
Cescato R, Schulz S, Waser B et al (2006) Internalization of sst2, sst3, and sst5 receptors: effects of somatostatin agonists and antagonists. J Nucl Med 47:502–511
Chaturvedi S, Mishra AK (2016) Small Molecule Radiopharmaceuticals—a review of current approaches. Front Med 3:1–18. doi:10.3389/fmed.2016.00005
De Lemos Pinto MMP, Santos NFG, Amaral A (2010) Current status of biodosimetry based on standard cytogenetic methods. Radiat Environ Biophys 49:567–581. doi:10.1007/s00411-010-0311-3
Demarini DM (2013) Genotoxicity biomarkers associated with exposure to traffic and near-road atmospheres: a review. Mutagenesis 28:485–505. doi:10.1093/mutage/get042
Deppen SA, Liu E, Blume JD et al (2016) Safety and efficacy of 68Ga-DOTATATE PET/CT for diagnosis, staging and treatment management of neuroendocrine tumors. J Nucl Med 57:708–715. doi:10.2967/jnumed.115.163865
Elhajouji A, Lukamowicz M, Cammerer Z, Kirsch-Volders M (2011) Potential thresholds for genotoxic effects by micronucleus scoring. Mutagenesis 26:199–204. doi:10.1093/mutage/geq089
Fenech M (2000) The in vitro micronucleus technique. Mutat Res Fundam Mol Mech Mutagen 455:81–95. doi:10.1016/S0027-5107(00)00065-8
Fenech M (2007) Cytokinesis-block micronucleus cytome assay. Nat Protoc 2:1084–1104. doi:10.1038/nprot.2007.77
Fenech M, Kirsch-Volders M, Rossnerova A et al (2013) HUMN project initiative and review of validation, quality control and prospects for further development of automated micronucleus assays using image cytometry systems. Int J Hyg Environ Health 216:541–552. doi:10.1016/j.ijheh.2013.01.008
Ferguson LR, Fenech MF (2012) Vitamin and minerals that influence genome integrity, and exposure/intake levels associated with DNA damage prevention. Mutat Res Fundam Mol Mech Mutagen 733:1–3. doi:10.1016/j.mrfmmm.2012.03.009
Harapanhalli RS (2010) Food and drug administration requirements for testing and approval of new radiopharmaceuticals. Semin Nucl Med 40:364–384. doi:10.1053/j.semnuclmed.2010.05.002
Heddle JA, Fenech M, Hayashi M, MacGregor JT (2011) Reflections on the development of micronucleus assays. Mutagenesis 26:3–10. doi:10.1093/mutage/geq085
IAEA (2007) Comparative evaluation of therapeutic radiopharmaceuticals. IAEA, Vienna
Johnbeck CB, Knigge U, Kjær A (2014) PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol 10:2259–2277. doi:10.2217/fon.14.139
Kashino G, Hayashi K, Douhara K et al (2014) Comparison of the biological effects of (18)F at different intracellular levels. Biochem Biophys Res Commun 454:7–11. doi:10.1016/j.bbrc.2014.09.136
Liu C, Gu Y (2013) Noninvasive optical imaging of Staphylococcus aureus infection in vivo using an antimicrobial peptide fragment based near-infrared fluorescent probes. J Innov Opt Health Sci 06:1350026. doi:10.1142/S1793545813500260
Lupetti A, de Boer MG, Erba P et al (2011) Radiotracers for fungal infection imaging. Med Mycol 49:S62–S69. doi:10.3109/13693786.2010.508188
Naga MBSS (2016) Buccal micronucleus cytome assay in sickle cell disease. J Clin Diagn Res 10:ZC62–4. doi:10.7860/JCDR/2016/19984.7998
Nair-Shalliker V, Armstrong BK, Fenech M (2012) Does vitamin D protect against DNA damage? Mutat Res Fundam Mol Mech Mutagen 733:50–57. doi:10.1016/j.mrfmmm.2012.02.005
Nersesyan A, Kundi M, Fenech M et al (2014) Micronucleus assay with urine derived cells (UDC): a review of its application in human studies investigating genotoxin exposure and bladder cancer risk. Mutat Res Rev Mutat Res 762:37–51. doi:10.1016/j.mrrev.2014.04.004
Nilica B, Waitz D, Stevanovic V et al (2016) Direct comparison of 68Ga-DOTA-TOC and 18F-FDG PET/CT in the follow-up of patients with neuroendocrine tumour treated with the first full peptide receptor radionuclide therapy cycle. Eur J Nucl Med Mol Imaging 43(9):1585–1592. doi:10.1007/s00259-016-3328-2
OECD (2010) Oecd guideline for the testing of chemicals: invitro mammalian cell micronucleus test. OECD, Paris
Okarvi SM (2008) Peptide-based radiopharmaceuticals and cytotoxic conjugates: potential tools against cancer. Cancer Treat Rev 34:13–26. doi:10.1016/j.ctrv.2007.07.017
Ostovar A, Assadi M, Vahdat K et al (2013) A pooled analysis of diagnostic value of (99 m)Tc-ubiquicidin (UBI) scintigraphy in detection of an infectious process. Clin Nucl Med 38:413–416. doi:10.1097/RLU.0b013e3182867d56
Polard T, Jean S, Merlina G et al (2011) Giemsa versus acridine orange staining in the fish micronucleus assay and validation for use in water quality monitoring. Ecotoxicol Environ Saf 74:144–149. doi:10.1016/j.ecoenv.2010.08.005
Salimi M, Broumand B, Mozdarani H (2016) Association of elevated frequency of micronuclei in peripheral blood lymphocytes of type 2 diabetes patients with nephropathy complications. Mutagenesis. doi:10.1093/mutage/gew029
Santos GS, Tsutsumi S, Vieira DP et al (2014) Effect of Brazilian propolis (AF-08) on genotoxicity, cytotoxicity and clonogenic death of Chinese hamster ovary (CHO-K1) cells irradiated with 60Co gamma-radiation. Mutat Res Genet Toxicol Environ Mutagen 762:17–23. doi:10.1016/j.mrgentox.2013.11.004
Sobol Z, Homiski ML, Dickinson DA et al (2012) Development and validation of an in vitro micronucleus assay platform in TK6 cells. Mutat Res Genet Toxicol Environ Mutagen 746:29–34. doi:10.1016/j.mrgentox.2012.02.005
Speit G, Zeller J, Neuss S (2011) The in vivo or ex vivo origin of micronuclei measured in human biomonitoring studies. Mutagenesis 26:107–110. doi:10.1093/mutage/geq061
Tian X-L, Zhao H, Cai T-J et al (2016) Dose-effect relationships of nucleoplasmic bridges and complex nuclear anomalies in human peripheral lymphocytes exposed to 60Co γ-rays at a relatively low dose. Mutagenesis 31:425–431. doi:10.1093/mutage/gew001
van Es SC, Venema CM, Glaudemans AWJM et al (2016) Translation of new molecular imaging approaches to the clinical setting: bridging the gap to implementation. J Nucl Med 57:96S–104S. doi:10.2967/jnumed.115.157974
Westerink WMA, Schirris TJJ, Horbach GJ, Schoonen WGEJ (2011) Development and validation of a high-content screening in vitro micronucleus assay in CHO-k1 and HepG2 cells. Mutat Res Genet Toxicol Environ Mutagen 724:7–21. doi:10.1016/j.mrgentox.2011.05.007
Acknowledgments
Ivette Zegarra Ocampo was a National Council for Scientific and Technological Development (CNPq) fellow (130778/2014-1). Camila Ayala Lira da Cruz was a PIBIC/PROBIC (CNPq) fellow (161411/2014-2). The authors wish to thank Drs. Elaine Bortoleti de Araújo e Maria Teresa Coulturato (IPEN—Center of Radiopharmacy—Quality Control Management) for valuable help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ocampo, I.Z., de Queiroz Souza Passos, P., Ramirez de Carvalho, L. et al. In vitro cytotoxic and genotoxic evaluation of peptides used in nuclear medicine (DOTATATE and Ubiquicidin29-41) in CHO-K1 cells. Cytotechnology 68, 2301–2310 (2016). https://doi.org/10.1007/s10616-016-0024-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-016-0024-9