Abstract
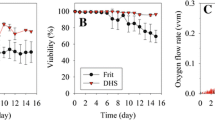
In this study, we evaluated three cell retention devices, an alternating tangential flow (ATF) system, a spin-filter, and a Centritech Lab III centrifuge, for the production of recombinant human Factor VIII co-expressed with von Willebrand factor. From the results, it was found that the FVIII activity in bioreactor was significantly higher in the ATF perfusion culture than two other perfusion cultures. Moreover, the FVIII activity yield was unexpectedly low in the ATF perfusion culture. We have, therefore, studied the reasons for this low FVIII activity yield. It was revealed that the inactivation and the surface adsorption of FVIII onto the harvest bag were not the main reasons for the low yield in the ATF perfusion culture. The FVIII activity yield was not increased by the use of a hollow fiber filter with 0.5 μm pore size instead of 0.2 μm pore size. Additionally, the retention of FVIII molecules by the hollow fiber filter was a dominant factor in the low FVIII activity yield in the ATF perfusion culture. We demonstrated that FVIII yield was significantly improved by controlling transmembrane pressure (TMP) across the hollow fiber filter membrane. Taken together, these results suggest that TMP control could be an efficient method for the enhancement of FVIII yield in an ATF perfusion culture.




Similar content being viewed by others
References
Berntorp E (1997) Second generation, B-domain deleted recombinant factor VIII. Thromb Haemost 78:256–260
Boedeker BG (2001) Production processes of licensed recombinant factor VIII preparations. Semin Thromb Hemost 27:385–394
Casademunt E, Martinelle K, Jernberg M, Winge S, Tiemeyer M, Biesert L, Knaub S, Walter O, Schroder C (2012) The first recombinant human coagulation factor VIII of human origin: human cell line and manufacturing characteristics. Eur J Haematol 89:165–176
Clincke MF, Molleryd C, Zhang Y, Lindskog E, Walsh K, Chotteau V (2013) Very high density of CHO cells in perfusion by ATF or TFF in WAVE bioreactor. Part I. Effect of the cell density on the process. Biotechnol Prog 29:754–767
Crowley J, Wubben M, Martin JMC (2012) Process for cell culturing by continuous perfusion and alternating tangential flow. US Patent 8206981 B1
Himmelfarb P, Thayer PS, Martin HE (1969) Spin filter culture: the propagation of mammalian cells in suspension. Science 164:555–557
Hoyer LW, Shainoff JR (1980) Factor VIII-related protein circulates in normal human plasma as high molecular weight multimers. Blood 55:1056–1059
Ishaque A, Thrift J, Murphy JE, Konstantinov K (2007) Over-expression of Hsp70 in BHK-21 cells engineered to produce recombinant factor VIII promotes resistance to apoptosis and enhances secretion. Biotechnol Bioeng 97:144–155
Jiang R, Monroe T, McRogers R, Larson PJ (2002) Manufacturing challenges in the commercial production of recombinant coagulation factor VIII. Haemophilia 8(Suppl 2):1–5
Johnson M, Lanthier S, Massie B, Lefebvre G, Kamen AA (1996) Use of the Centritech Lab centrifuge for perfusion culture of hybridoma cells in protein-free medium. Biotechnol Prog 12:855–864
Kaufman RJ, Pipe SW (1999) Regulation of factor VIII expression and activity by von Willebrand factor. Thromb Haemost 82:201–208
Kaufman RJ, Wasley LC, Dorner AJ (1988) Synthesis, processing, and secretion of recombinant human factor VIII expressed in mammalian cells. J Biol Chem 263:6352–6362
Kaufman RJ, Wasley LC, Davies MV, Wise RJ, Israel DI, Dorner AJ (1989) Effect of von Willebrand factor coexpression on the synthesis and secretion of factor VIII in Chinese hamster ovary cells. Mol Cell Biol 9:1233–1242
Kelley B, Jankowski M, Booth J (2010) An improved manufacturing process for Xyntha/ReFacto AF. Haemophilia 16:717–725
Kim BJ, Oh DJ, Chang HN (2008) Limited use of Centritech Lab II centrifuge in perfusion culture of rCHO cells for the production of recombinant antibody. Biotechnol Prog 24:166–174
McLeod A, Walker I, Zheng S, Hayward C (2000) Loss of factor VIII activity during storage in PVC containers due to adsorption. Haemophilia 6:89–92
Mei B, Chen Y, Chen J, Pan CQ, Murphy JE (2006) Expression of human coagulation factor VIII in a human hybrid cell line, HKB11. Mol Biotechnol 34:165–178
Mercille S, Johnson M, Lemieux R, Massie B (1994) Filtration-based perfusion of hybridoma cultures in protein-free medium: reduction of membrane fouling by medium supplementation with DNase I. Biotechnol Bioeng 43:833–846
Mufarrege EF, Antuña S, Etcheverrigaray M, Kratje R, Prieto C (2014) Development of lentiviral vectors for transient and stable protein overexpression in mammalian cells. A new strategy for recombinant human FVIII (rhFVIII) production. Protein Expr Purif 95:50–56
Selvaraj SR, Scheller AN, Miao HZ, Kaufman RJ, Pipe SW (2012) Bioengineering of coagulation factor VIII for efficient expression through elimination of a dispensable disulfide loop. J Thromb Haemost 10:107–115
Seyfried BK, Friedbacher G, Rottensteiner H, Schwarz HP, Ehrlich H, Allmaier G, Turecek PL (2010) Comparison of plasma-derived and recombinant von Willebrand factor by atomic force microscopy. Thromb Haemost 104:523–530
Shankaran H, Alexandridis P, Neelamegham S (2003) Aspects of hydrodynamic shear regulating shear-induced platelet activation and self-association of von Willebrand factor in suspension. Blood 101:2637–2645
Shevitz J (2003) Fluid filtration system. US 6544424 B1
Tokashiki M, Arai T, Hamamoto K, Ishimaru K (1990) High density culture of hybridoma cells using a perfusion culture vessel with an external centrifuge. Cytotechnology 3:239–244
Vlot AJ, Koppelman SJ, van den Berg MH, Bouma BN, Sixma JJ (1995) The affinity and stoichiometry of binding of human factor VIII to von Willebrand factor. Blood 85:3150–3157
Ward NJ, Buckley SM, Waddington SN, VandenDriessche T, Chuah MK, Nathwani AC, McIntosh J, Tuddenham EG, Kinnon C, Thrasher AJ (2011) Codon optimization of human factor VIII cDNAs leads to high-level expression. Blood 117:798–807
Weiss HJ, Sussman II, Hoyer LW (1977) Stabilization of factor VIII in plasma by the von Willebrand factor. Studies on posttransfusion and dissociated factor VIII and in patients with von Willebrand’s disease. J Clin Invest 60:390–404
Wise RJ, Dorner A, Krane M, Pittman D, Kaufman R (1991) The role of von Willebrand factor multimers and propeptide cleavage in binding and stabilization of factor VIII. J Biol Chem 266:21948–21955
Yabannavar VM, Singh V, Connelly NV (1992) Mammalian cell retention in a spinfilter perfusion bioreactor. Biotechnol Bioeng 40:925–933
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, SC., An, S., Kim, HK. et al. Effect of transmembrane pressure on Factor VIII yield in ATF perfusion culture for the production of recombinant human Factor VIII co-expressed with von Willebrand factor. Cytotechnology 68, 1687–1696 (2016). https://doi.org/10.1007/s10616-015-9918-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-015-9918-1