Abstract
This study investigated the in vitro growth characteristics and differential potential of muscle-derived satellite cells (MDSCs) derived from rats at different postnatal (P) stages, in order to expand the range of source material for tissue engineering. Rat MDSCs were isolated from P5, P10, P15, P21 and P42 rat skeletal muscles using double enzyme digestion and differential adherent culture. Neurogenic, osteogenic and myogenic induction media were used to induce directed differentiation. Differentiated nerve cells, osteoblasts and myotubes were identified by their morphology and immunohistochemical staining. Most cells transformed into spindle-shaped mononuclear cells after 48 h and proliferated rapidly. MDSCs were difficult to isolate from P42 rats. After neurogenesis, four groups MDSCs formed neuron-specific enolase positive polygonal-shaped dendritic cells. After osteogenesis, P5, P10, P15 and P21 MDSCs formed Alizarin red- and osteocalcin-positive bone nodules. After myogenesis, myotubes were formed and were fast muscle myosin-positive. MDSCs derived from P5, P10, P15 and P21 rat skeletal muscle are easy to isolate, culture and amplify in vitro, which increases the range of source material available for tissue engineering.
Similar content being viewed by others
Introduction
As the population of China becomes older, age-related diseases have increasingly become a major concern in society. Sarcopenia is one such age-related disease, which is named after the Greek meaning “poverty of flesh”. The term was first introduced by Irwin et al. in 1989 to describe age-related reduction in muscle and strength (Irwin et al. 1989). In 2010, the European Working Group on Sarcopenia in Older People developed a practical definition of sarcopenia as the presence of both low muscle mass and low muscle function (strength or performance) (Cruz-Jentoft et al. 2010).
Cachexia is a muscle-related disease similar to sarcopenia. Cachexia is a metabolic condition which is always associated with an underlying illness or inflammation. In the early stages, cachexia has no obvious symptoms; however, the patients continuously lose weight and undergo muscle wastage in the later stages. The symptoms of cachexia include anorexia, long-term nausea, constipation, weakness, depression and changes in physical appearance and the disease can lead to paralysis and even death (Evans et al. 2008; Tisdale 2002). Abnormal changes in muscle metabolism occur during both cachexia and sarcopenia, indicating that a similar molecular mechanism underlies the process of muscle atrophy in both conditions.
Although numerous studies have attempted to reduce muscle atrophy and have resulted in a partial remission of this process (Grounds and Davies 2007), these measures do not fundamentally solve the problem of how to recover the function of atrophied muscle. With the rapid development of cell biology and the rise of tissue engineering in recent years, the transplantation of muscle-derived satellite cells (MDSCs) has been considered to be the best treatment for muscle atrophy (Grounds and Davies 2007). However, the transplanted cells are vulnerable to attack by the host immune system and may not survive long term, and the overall effects of MDSC transplantation are poor, which highlights immune rejection as an important issue in cell therapy (Crisan et al. 2008). The better purified myoblasts led to an augmentation of transplantation efficiency (Qu and Huard 2000). Günther and Walter (2001) found that highly purified MDSCs expressing both type I and type II major histocompatibility complex could completely avoid rapid cell death after transplantation, indicating that purification of MDSCs can prevent immune rejection. The proportion of MDSCs in skeletal muscle cells is low, approximately 1–5 % (Kuang et al. 2007). Therefore, in order to further research into the treatment of myopathy, it is important to develop techniques which enable the purification of sufficient numbers of MDSCs.
MDSCs isolated from the patients’ own skeletal muscle can potentially be used for the reconstruction of skeletal and/or cardiac muscle in clinical practice (Arsic et al. 2008; Nolazco et al. 2008; Tamaki et al. 2008; Beauchamp et al. 2000), which avoids the issues associated with immune rejection when using donor cells. The number of MDSCs in muscle gradually decreases to a certain level with the increase of age, and is then maintained for life. In the period after birth, the proportion of satellite cell nuclei decreases significantly. As the fusion of MDSCs occurs, the total number of muscle cell nuclei significantly increases, resulting in a net decrease of MDSCs (Rossello and Kohn 2009; Gibson and Schultz 1983). Therefore, further research on the isolation and in vitro culture of MDSCs is crucial for the development of novel treatments for muscle disease.
In this study, we abandoned the long held idea that MDSCs can only be isolated from rat skeletal muscle within 10 days after birth (Day et al. 2010). We explored the in vitro growth, proliferation and differentiation capacity of MDSCs isolated from rat skeletal muscle up to 42 days after birth, in order to provide a more substantial basis to identify whether MDSCs are ideal seed cells for regenerative medicine and explore their clinical applications. The study of cells cultured in stents is an indispensable, important aspect of tissue engineering (Sacco et al. 2008). Hence, we also investigated the ability of the isolated MDSCs to adhere and grow on polylactic-co-glycolic acid (PLGA) stents.
Materials and methods
Reagents
Type I collagenase and dimethylsulfoxid (DMSO) were purchased from Sigma (St. Louis, MO, USA). Trypsin and DMEM/F12 were obtained from Invitrogen (Carlsbad, CA, USA). All antibodies were purchased from Abcam (Cambridge, U.K.).
Animals
Postnatal day (P) 5, 10, 15, 21 and 42 Wistar rats and their mothers had free access to water and standard rat chow. All studies were performed with the approval of the Experimental Animal Committee at our university.
Isolation of MDSCs
MDSCs were isolated from the leg muscle of P5, P10, P15, P21 and P42 rats according to a previously described method, with some modifications (Dorfman et al. 1998; Gharaibeh et al. 2008). Briefly, the muscles were removed from euthanized rats under sterile conditions, soaked in 75 % alcohol for 20 min, washed with phosphate buffer solution (PBS) containing penicillin/streptomycin (15140-122; Invitrogen Corp.), cut into 1–2 mm2 fragments, digested with 0.1 % type I collagenase (C0130; Sigma–Aldrich) or 0.1 % type IV collagenase (Sigma-Aldrich) in PBS for 1 h at 37 °C in a shaking water bath, centrifuged at 150 g for 5 min and the supernatant was discarded. The pellet was resuspended in 0.25 % trypsin, incubated for 20 min at 37 °C, FBS (Fetal Bovine Serum) (12664-025; Invitrogen Corp.) was added, the mixture was centrifuged and the pellet was resuspended in growth medium [DMEM/F12 (Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 Ham) containing 20 % FBS, 10 % HS (Horse Serum)(26050-088; Invitrogen Corp.) and 1 % PS (penicillin/streptomycin)]. After repeated pipetting, the cells were passed through a 200-mesh sieve and subjected to successive centrifugation (150 g for 5 min). Cell viability was assessed using trypan blue staining.
Purification and culture of MDSCs
The MDSCs were purified using the preplate technique, as previously described (Gharaibeh et al. 2008). Briefly, the cells were cultured for 2 h in 6-well plates which had been precoated with glutin (53028; Sigma–Aldrich). The adherent cells were named P0P1, and the non-adherent cells were removed, seeded into a different 6-well plate and cultured for 12 h. These adherent cells were named P0P2, and the non-adherent cells were removed, seeded into a different 6-well plate, cultured for 24 h and the adherent cells were named P0P3. The P0P1, P0P2 and P0P3 cells were cultured without removing the medium for 3 days to ensure adhesion, then the medium was replaced every 2 days and the cells were sub-cultured when 80 % confluent. Different combinations of serum (10 % FBS, 20 % FBS, 10 % HS, 20 % HS and 20 % FBS + 10 % HS) were tested to investigate the effect of tissue age and serum on MDSCs culture.
MDSCs growth curve
MDSCs derived from P5, P10, P15 and P21 rat skeletal muscle at passage 30 were used for establishing growth curves. Cells were adjusted to 1 × 104 cells/mL and seeded in 24-well plates. Beginning the next day, cells were harvested from three wells for cell counting, continuing each day to generate a growth curve. After 8 days, this growth curve was generated.
Immunostaining
MDSCs were identified by immunostaining, as previously described (Elabd et al. 2007). Briefly, fifth passage MDSCs isolated from P5, P10, P15 and P21 rats were seeded into 24-well plates (1 × 105 cells/mL), cultured to 80 % confluence, fixed with 4 % paraformaldehyde for 12 h, permeabilized with PBS containing 0.1 % (vol/vol) Triton X-100 (Sigma-Aldrich, St.Louis, MO) for 2 h, incubated with 3 % Bovine serum albumin (A1933; Sigma–Aldrich) for 2 h followed by 3 % bovine serum albumin (BSA) (A2058; Sigma–Aldrich) in PBS for 2 h. Primary mouse anti-desmin, mouse anti-α-sarcomeric actinin, mouse anti-myod1, mouse anti-myf5 or mouse anti-pax7 antibodies (1:200) were applied, incubated for 12 h at 4 °C followed by incubation with FITC-labeled secondary antibodies (1:100) for 2 h, then the cells were stained with DAPI [4′-6-diamidino-2-phenylindole, (Sigma-Aldrich, St.Louis, MO)]. PBS was used in place of the primary antibody as a negative control; staining was visualized by fluorescent microscopy.
Induction and confirmation of MDSCs differentiation into neural cells
Four groups of rat MDSCs were pre-induced with DMEM/F12 supplemented with 10 ng/mL EGF and 10 ng/mL bFGF for 24 h. The medium was replaced with DMEM/F12 supplemented with 5 mM β-mercaptoethanol (21985-023, Gibco, Gaithersburg, MD, USA) and 10 % FBS, the cells were cultured for 6 h, washed and fresh DMEM/F12 supplemented with 2 % DMSO, 200 μM Butyl Hydroxy Anisd (20021; Sigma–Aldrich) and 20 ng/ml bFGF (G5071, Promega, Madison, WI, USA) was added. The control cells were cultured in DMEM/F12 containing 20 % FBS. The cells were observed every 1 h under a microscope, and neuronal cells were identified by neuron-specific enolase (NSE) staining, as previously described (Hermann et al. 2006).
Induction and confirmation of MDSCs differentiation into osteoblasts
Fifth passage MDSCs isolated from P5, P10, P15 and P21 rats were seeded into 6-well plates (1 × 105 cells/mL), cultured to 80 % confluence and the medium was replaced with osteoblast induction medium [DMEM/F12 supplemented with 10 % FBS, 10 mmol/L β-sodium glycerophosphate (50020; Sigma–Aldrich), 20 nmol/L dexamethasone (D4902; Sigma–Aldrich) and 50 μg/mL ascorbic acid (A2218; Sigma–Aldrich)). The medium was changed every 3 days and differentiation was confirmed 21 days later by Alizarian red staining (Ren et al. 2000). Cells were first washed 3 times with 150 mM NaCl then were in 70 % ethanol for 1 hour at 4 °C, at last 2 % alizarin red stained for 10 minutes at room temperature. Mineral deposition was detected under phase contrast microscopy. Synthesis of the bone-specific protein osteocalcin was determined using a DAB (3,3N-Diaminobenzidine Tertrahydrochloride) assay, as previously described (Elabd et al. 2007).
Induction and confirmation of MDSCs differentiation into myoblasts
Fifth passage MDSCs isolated from P5, P10, P15 and P21 rats were seeded into 6-well plates (1 × 105 cells/mL), cultured to 80 % confluence and the medium was replaced with myoblast induction medium (DMEM/F12 supplemented with 10 % HS). When thick short or narrow long club-shaped cells were observed, the cells were stained with 5 mg/L Hoechst 33342 (Sigma-Aldrich, St. Louis, MO) to detect cell fusion. The cells were also fixed and stained using an antibody to detect myosin, a marker of muscle differentiation, as previously described (Chang et al. 2012).
MDSCs growth on PLGA-based scaffolds
PLGA sheets (P2066; Sigma–Aldrich) were sterilized, as described (Lee et al. 2007). Fifth passage MDSCs isolated from P5, P10, P15 and P21 rats were seeded onto the PLGA at a density of 4 × 105 cells/mL. When the cells grew to 80 % confluence, the cells were digested using 0.05 % trypsin to investigate whether the cells detached easily from the PLGA.
Results
Morphological observations and culture
The skeletal muscle tissues of P5, P10, P15, P21 and P42 rats were subjected to a double enzyme digestion method. Muscle bundle digestion was more rapid with type I collagenase than type IV collagenase, and trypsin was subsequently used to obtain single cells; however, the trypsin digestion time should be as short as possible. Round MDSCs could be clearly observed in the cell suspensions. The cell suspensions from P5, P10, P15 and P21 rats skeletal muscle tissues contained a high number of small, rounded MDSCs and a low number of large, mature rod myotube cells (Fig. 1a–d), whereas the cell suspensions from P42 rat skeletal muscle tissues contained a high number of large, mature rod myotube cells and low number of small rounded MDSCs (Fig. 1e). Additionally, there were almost no adherent cells after incubation of P42 P2P0 and P3P0 cells for 18 h. Due to the difficulty of separating rat MDSCs from P42 rats, P42 skeletal muscle tissues are not an appropriate source for the separation of primary cells, and the MDSCs from P42 rats were not included in the subsequent experiments.
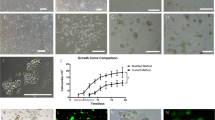
In the first differential culture of the cells obtained from P5, P10, P15 and P21 rats, the adherent P1P0 cells were immunohistochemically identified as mainly fiber cells and endothelial cells (data not shown); hence the P1P0 cells were discarded. The P2P0 and P3P0 cells obtained from P5, P10, P15 and P21 rats adhered after 8 h culture, and some of these cells developed small processes. The vast majority of P2P0 and P3P0 cells transformed into spindle-shaped mononuclear cells within 48 h, with abundant cytoplasm and a high cellular refractive index. The fusiform shape became more apparent as incubation continued; after 3 days incubation cell confluency reached 80 % and the cells were passaged to obtain high purity rat MDSCs from P2P0 and P3P0 cells (Fig. 2).
Morphology of P0P3 MDSCs isolated from rats at different postnatal stages (P) using the differential adherence culture method. a–d MDSCs isolated from P5 rats (a), P10 rats (b), P15 rats (c) and P21 rats (d). The vast majority of P0P2 and P0P3 cells transformed into spindle-shaped mononuclear cells after 48 h culture, with abundant cytoplasm and a high cell refractive index. The size of the scale bar is 100 μm
DMEM/F12 containing 10 % FBS, 20 % FBS, 10 % HS, 20 % HS and 20 % FBS + 10 % HS was used to culture the P2P0 and P3P0 MDSCs obtained from P5, P10, P15 and P21 rats, to investigate the effects of tissue age and serum on the culture of MDSCs. In 20 % FBS medium, the second passage cells initially grew vigorously. Over the next few days, a number of rod-like cells with a high refractivity appeared, then the length and quantity of rod-shaped cells gradually increased; the volume of these cells was significantly larger than a single MDSC. Nuclei could be clearly observed at the swollen part of cytoplasm, indicating the formation of myotube cells. The rate of differentiation of cells cultured in FBS-containing media varied and the growth rate of cells cultured in 10 % FBS medium was slower than cells cultured in 20 % FBS medium. The cells cultured in different concentrations of HS-containing medium underwent a similar pattern of growth but slower than cells cultured in FBS media. Therefore, 20 % FBS + 10 % HS-containing medium was selected as the in vitro growth medium for MDSCs for all subsequent experiments (data not shown).
Growth of MDSCs
Thirtieth passage MDSCs obtained from P5, P10, P15 and P21 rats entered exponential growth phase between day 2 and 3, and reached a growth plateau between day 6 and 7. The growth rate of four kinds of MDSCs was not significantly different (P > 0.05) (Fig. 3).
Identification of MDSCs by immunohistochemistry
Positive desmin, α-sarcomeric actinin, MyoD1, Myf5 and PAX7 immunostaining were observed to be densely distributed in the nucleus, while the DAPI-positive nuclei were immunonegative (Fig. 4).
Immunohistochemical identification of fifth passage MDSCs isolated from rats at different postnatal (P) days. Desmin, α-sarcomeric actinin, MyoD1, Myf5 and PAX7 immunostaining (all positive; green), respectively from left to right, of MDSCs isolated from P5 rats (a–e), P10 rats (g–k), P15 rats (m–q) and P21 rats (s–w). f, l, r and x are the corresponding negative control cells (PBS was used instead of a primary antibody). All images are ×100; nuclei are counterstained with DAPI (blue). The size of the scale bar is 100 μm
Induction of neurogenesis in MDSCs
After pre-induction for 24 h and formal induction for 6 h, MDSCs did not undergo obvious morphological changes. After 2 h, the cell bodies became conical, triangular or round in shape with multiple protrusions resembling axons, the ends of which were primary and secondary bifurcated dendrites. For control group showed no obvious morphological changes. Immunohistochemical analysis demonstrated that the differentiated cells all expressed NSE at 2 h after induction (Fig. 5a1–d1); the control (non-induced) groups did not express NSE (Fig. 5a2–d2).
Differentiation of MDSCs isolated from rats at different postnatal (P) days into nerve cells. a–d are morphologic changes after neurogenic differentiation in bright field. a1–d1 Formation of obvious axonal and dendritic structures, as indicated by Cy3 staining (red) after the induction of neurogenesis in fifth passage MDSCs isolated from P5 rats (a1), P10 rats (b1), P15 rats (c1) and P21 rats (d1); a2–d2 are the uninduced control cells corresponding to a1–d1. All images are x100; nuclei are counterstained with DAPI (blue). The size of the scale bar is 100 μm
Osteogenic induction
During osteogenic induction, MDSCs obtained from P5, P10, P15 and P21 rats formed obvious bone nodules, as indicated by Alizarin red staining. At the initial stage of induction, the MDSCs displayed monoclonal-like growth and changed from spindle-shaped to irregularly-shaped cells. On the eighteenth day of induction, typical monoclonal cells appeared and the cells began to gather. After the twenty-first day of induction, the cells aggregated and formed obvious knots of ossification, which could be stained bright red using Alizarin red (Fig. 6a–d). When seeded at the same density, the number of bone nodules formed under osteogenic induction by MDSCs obtained from P15 and P21 rats was greater than the MDSCs obtained from P5 and P10 rats (Table 1.). Immunohistochemical staining demonstrated that osteocalcin was expressed in the osteogenic cells differentiated from MDSCs obtained from P5, P10, P15 and P21 rats (Fig. 6a1–d1); no expression of osteocalcin was detected in the control (non-induced) groups (Fig. 6a2–d2). These results indicated that MDSCs obtained from P5, P10, P15 and P21 rats could be induced to differentiate into osteoblasts.
Osteogenic differentiation of MDSCs isolated from rats at different postnatal (P) days. a–d Obvious formation of bone nodules, as indicated by Alizarin red staining, after osteogenic induction of fifth passage MDSCs isolated from P5 rats (a), P10 rats (b), P15 rats (c) and P21 rats (d). a1–d1 Positive expression of osteocalcin (DAB staining; yellow) after osteogenic induction of fifth passage MDSCs isolated from P5 rats (a1), P10 rats (b1), P15 rats (c1) and P21 rats (d1); a2–d2 are the uninduced control cells corresponding to a1–d1. All images are ×100. The size of the scale bar is 100 μm
Myogenic induction
At 72 h after the addition of myogenic induction medium, the MDSCs obtained from P15 and P21 rats began to fuse, and gradually and regularly arranged in parallel to each other in a single direction to form short, thick, multicore myotube cells. As time passed, cell density increased, the integration between the cells became more extensive, and the number and length of the myotube cells increased markedly (Fig. 7c, d). MDSCs obtained from P5 and P10 rats began to integrate 5 days after myogenic induction, leading to obvious myotube cell formation (Fig. 7a, b). DAPI staining revealed the presence of multiple nuclei in the same myotube, and immunohistochemical staining demonstrated the cells were fast muscle myosin-positive (Fig. 7a1–d1); the cells of the control (non-induced) groups were fast muscle myosin negative (Fig. 7a2–d2). These results indicated that MDSCs obtained from P5, P10, P15 and P21 rats could be induced to differentiate into myogenic cells.
Myogenic differentiation of MDSCs isolated from rats at different postnatal (P) days. a1–d1 Formation of myotubes after myogenic induction of fifth passage MDSCs isolated from P5 rats (a), P10 rats (b), P15 rats (c) and P21 rats (d), as indicated by positive immunostaining for fast muscle myosin (Cy3 staining; red). a2–d2 are the uninduced control cells corresponding to a1–d1. All images are ×100; nuclei are counterstained with DAPI (blue). The size of the scale bar is 100 μm
Attached growth of rat MDSCs on PLGA
Six hours after the cell suspensions were added, MDSCs obtained from P5, P10, P15 and P21 rats began to adhere to the PLGA carrier. After 2 days, adhesive cell growth along the stent fiber could be observed using DAPI staining. The medium was replaced with fresh medium every other day, and when the cells covered 90 % of the PLGA surface (Fig. 8), 0.05 % trypsin was added, incubated for 1 min at 37 °C and the non-adherent cells were collected by repeated blowing and suction with a pipette, followed by centrifugation (150 g for 5 min). A similar number of cells were collected from each group. As culture continued, the trypsinised PLGA inserts began to degrade at week four and completely degraded in the eighth week. These results suggested that MDSCs obtained from P5, P10, P15 and P21 rats could adhere and grow on PLGA, with no significant differences in the rate of cell proliferation rate or morphology during their growth on PLGA. Additionally, the MDSCs could be easily collected from PLGA and are therefore suitable as seed cells for transplantation during tissue engineering.
Proliferation of MDSCs isolated from rats at different postnatal days (P) on PLGA. Growth of fifth passage MDSCs isolated from P5 rats (a), P10 rats (b), P15 rats (c) and P21 rats (d) on PLGA. Cells are viewed using the bright field green filter (a–d) and UV filter to visualize Hoechst 33342 nuclear staining (a1–d1); all images are ×100. The size of the scale bar is 100 μm
Discussion
The choice of optimal seed cells is crucial during tissue engineering. At present, the major seed cells used are embryonic stem cells, bone marrow mesenchymal stem cells (MSCs), umbilical cord blood MSCs and adipose MSCs. Embryonic stem cells can be induced to differentiate into various tissue cells; however, there are significant ethical issues which limit their practical application (Wobus and Boheler 2005). It is generally believed that bone marrow MSCs are an ideal seed cell for tissue engineering; however, MSCs are very rare, even in the bone marrow. Additionally, bone marrow biopsy induces significant trauma and pain to the patients, and only a limited volume of bone marrow can be collected. Therefore MSCs are not conducive for clinical applications on a large scale. The sources of umbilical cord blood MSCs are limited, and the cells are difficult to isolate and expensive to preserve, which limits the potential of these cells in clinical applications. There are rich sources of adipose MSCs and their separation is relatively easy; however, a smaller number of adipose MSCs can be isolated from adipose tissue compared to the number of MDSCs separated from skeletal muscle tissue of the same weight, and adipose MSCs grow more slowly than MDSCs (Guilak et al. 2006). Humans have a rich supply of muscle tissue which can be harvested easily, and a large number of MDSCs can be isolated from a small quantity of tissue. MDSCs have been confirmed to have the potential to differentiate into a variety of mesoderm and ectoderm-derived cells (Baksh et al. 2004). As skeletal muscle is a rich source of MDSCs and autologous transplantation can avoid many ethical and technical issues, MDSCs have become viewed as the ideal seed cells for tissue engineering.
A variety of methods have been reported for the separation and purification of skeletal muscle satellite cells, such as direct separation from the muscle bundle and isolation according to cell surface markers using flow cytometry and immunomagnetic separation (Jankowski et al. 2001). However, the desired effects are often not achieved and these in vitro procedures are complex and often become contaminated. Most research has been limited to skeletal muscle satellite cells derived from rats within 10 days after birth. In this study, MDSCs were separated from rats up to 42 days old using a simple double enzyme digestion and differential adherent culture method, which greatly expands the range of source material for tissue engineering research. Subsequently, the cells were classified on the basis of their time of attachment during the differential adherent culture method (Qu-Petersen et al. 2002). P2P0 and P3P0 cells were cultured to establish MDSCs, and the continuous culture of multiple generations of cells proved that the isolated cells had a high degree of self-renewal ability. Additionally, the growth rate of MDSCs derived from P5, P10, P15 and P21 rats did not significantly differ up to the thirtieth generation. Moreover, we observed that high concentrations of horse serum were necessary for the subculture of rat MDSCs; however, low concentrations of horse serum were conducive for the differentiation of rat MDSCs into myotubes (Wu et al. 2012).
Immunohistochemical staining for desmin, α-sarcomeric actinin, MyoD1, Myf5 and PAX7 confirmed that the cells were MDSCs. The progeny of satellite cells have different fates in culture. Desmin is intermediate filament protein, one ingredient of the cytoskeleton, which is specifically expressed in muscle fibers, and positive desmin immunostaining confirmed that the isolated cells were myogenic. Original satellite cells express the transcription factor PAX7, while activated satellite cells express both PAX 7 and MyoD (Huard et al. 2003). As activated satellite cells proliferate and differentiate, the expression of PAX 7 is downregulated, and the myogenic regulatory factors Myf5, MyoD, myogenin and MRF4 are only found in activated satellite cells, where they mediate the activation of skeletal muscle specialization genes and induce the differentiation of satellite cells (Seale and Rudnicki 2000).
MDSCs have a variety of potential applications in tissue engineering, such as the repair and reconstruction of skeletal muscle, cardiac muscle, bone, cartilage and peripheral nerves (Zammit et al. 2002; Seale and Rudnicki 2000; Deasy et al. 2008; Payne et al. 2007; Lee et al. 2007; Kornegay et al. 2010). In 1991, Law et al. (1991) transplanted MDSCs for the treatment of Duchenne muscular dystrophy for the first time. Although the overall effect was poor, MDSCs still demonstrated good potential for the treatment of this myopathy.
In this study, after culturing the MDSCs in neurogenesis induction medium, the cell membranes formed obvious axonal and dendritic structures, and the cells were NSE-positive, a characteristic of nerve cells. After culture in osteogenic induction medium, the MDSCs formed bone nodules and the osteoblast-specific protein osteocalcin was expressed, providing preliminary evidence that the MDSCs had the potential to differentiate into osteoblasts. Additionally, 2 % horse serum could induce the rat MDSCs to differentiate into myotubes. Taken together, these results indicate that the MDSCs isolated from P5, P10, P15 and P21 rats had a certain degree of pluripotency and were reliable somatic stem cell lines.
At present, there are several problems in MDSCs transplantation treatments, inducing the low survival rate of cells after direct transplantation (Nolazco et al. 2008). One method to solve this important problem in tissue engineering is to transplant a large number of cells after attachment to a scaffold material, however, this approach requires a rich source of cells. In previous studies, the research materials have been limited to skeletal muscle tissue harvested from rats within 10 days of birth (Day et al. 2010). This study demonstrates that MDSCs can be isolated from the skeletal muscle tissue of rats up to 3 weeks after birth, thus expanding the source material for MDSCs. The adherence of MDSCs to an appropriate material both in vivo or in vitro is essential for cell migration, proliferation and differentiation (Lee et al. 2007), and the ability of inoculated seed cells to proliferate on tissue engineering scaffolds is an important indicator of clinical feasibility (Collins et al. 2005). The tissue engineering scaffold material PLGA used in this study has a three-dimensional interlinked pore structure, which provides space for the attachment and proliferation of seed cells (Su et al. 2011; Makadia and Siegel 2011) and also enables efficient nutritional supply and metabolite discharge when the cells are cultured in vitro or grow in vivo. Our morphological observations indicated that the MDSCs derived from P5, P10, P15 and P21 rats adhered well and proliferated at a steady rate on PLGA scaffolds, suggesting that all four groups of rat MDSCs could take full advantage of the nutrients in the medium and secrete extracellular matrix.
Conclusion
Isolation of MDSCs from the skeletal muscle of rats up to 21 days old using the simple double enzyme digestion and differential adherent culture method can provide MDSCs with multi-directional differentiation potential, which can also adhere and grow on PGLA scaffolds. This study increases the range of source material available for research into suitable seed cells for tissue engineering and regenerative medicine.
References
Arsic N, Mamaeva D, Lamb NJ, Fernandez A (2008) Muscle-derived stem cells isolated as non-adherent population give rise to cardiac, skeletal muscle and neural lineages. Exp Cell Res 314:1266–1280. doi:10.1016/j.yexcr.2008.01.009
Baksh D, Song L, Tuan RS (2004) Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med 8:301–316
Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS (2000) Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol 151:1221–1234
Chang YF, Liu TY, Liu ST, Tseng CN (2012) Arecoline inhibits myogenic differentiation of C2C12 myoblasts by reducing STAT3 phosphorylation. Food Chem Toxicol Int J Publ British Ind Biol Res Assoc 50:3433–3439. doi:10.1016/j.fct.2012.07.032
Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE (2005) Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122:289–301. doi:10.1016/j.cell.2005.05.010
Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313. doi:10.1016/j.stem.2008.07.003
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39:412–423. doi:10.1093/ageing/afq034
Day K, Shefer G, Shearer A, Yablonka-Reuveni Z (2010) The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev Biol 340:330–343. doi:10.1016/j.ydbio.2010.01.006
Deasy BM, Schugar RC, Huard J (2008) Sex differences in muscle-derived stem cells and skeletal muscle. Crit Rev Eukaryot Gene Expr 18:173–188
Dorfman J, Duong M, Zibaitis A, Pelletier MP, Shum-Tim D, Li C, Chiu RC (1998) Myocardial tissue engineering with autologous myoblast implantation. J Thorac Cardiovasc Surg 116:744–751
Elabd C, Chiellini C, Massoudi A, Cochet O, Zaragosi LE, Trojani C, Michiels JF, Weiss P, Carle G, Rochet N, Dechesne CA, Ailhaud G, Dani C, Amri EZ (2007) Human adipose tissue-derived multipotent stem cells differentiate in vitro and in vivo into osteocyte-like cells. Biochem Biophys Res Commun 361:342–348. doi:10.1016/j.bbrc.2007.06.180
Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD (2008) Cachexia: a new definition. Clin Nutr 27:793–799. doi:10.1016/j.clnu.2008.06.013
Gharaibeh B, Lu A, Tebbets J, Zheng B, Feduska J, Crisan M, Peault B, Cummins J, Huard J (2008) Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc 3:1501–1509. doi:10.1038/nprot.2008.142
Gibson MC, Schultz E (1983) Age-related differences in absolute numbers of skeletal muscle satellite cells. Muscle Nerve 6:574–580. doi:10.1002/mus.880060807
Grounds MD, Davies KE (2007) The allure of stem cell therapy for muscular dystrophy. Neuromuscul Disord NMD 17:206–208. doi:10.1016/j.nmd.2007.01.007
Guilak F, Lott KE, Awad HA, Cao Q, Hicok KC, Fermor B, Gimble JM (2006) Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol 206:229–237. doi:10.1002/jcp.20463
Günther E, Walter L (2001) The major histocompatibility complex of the rat (Rattus norvegicus). Immunogenetics 53:520–542
Hermann A, Liebau S, Gastl R, Fickert S, Habisch HJ, Fiedler J, Schwarz J, Brenner R, Storch A (2006) Comparative analysis of neuroectodermal differentiation capacity of human bone marrow stromal cells using various conversion protocols. J Neurosci Res 83:1502–1514. doi:10.1002/jnr.20840
Huard J, Cao B, Qu-Petersen Z (2003) Muscle-derived stem cells: potential for muscle regeneration. Birth Defects Res Part C Embryo Today Rev 69:230–237. doi:10.1002/bdrc.10020
Irwin M, Jones L, Britton K, Hauger RL (1989) Central corticotropin releasing factor reduces natural cytotoxicity. Time course of action. Neuropsychopharmacology 2:281–284
Jankowski RJ, Haluszczak C, Trucco M, Huard J (2001) Flow cytometric characterization of myogenic cell populations obtained via the preplate technique: potential for rapid isolation of muscle-derived stem cells. Hum Gene Ther 12:619–628. doi:10.1089/104303401300057306
Kornegay JN, Li J, Bogan JR, Bogan DJ, Chen C, Zheng H, Wang B, Qiao C, Howard JF Jr, Xiao X (2010) Widespread muscle expression of an AAV9 human mini-dystrophin vector after intravenous injection in neonatal dystrophin-deficient dogs. Mol Ther J Am Soc Gene Ther 18:1501–1508. doi:10.1038/mt.2010.94
Kuang S, Kuroda K, Le Grand F, Rudnicki MA (2007) Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129:999–1010. doi:10.1016/j.cell.2007.03.044
Law PK, Goodwin TG, Fang QW, Chen M, Li HJ, Florendo JA, Kirby DS (1991) Myoblast transfer therapy for Duchenne muscular dystrophy. Acta Paediatrica Japonica 33:206–215 Overseas edition
Lee PY, Cobain E, Huard J, Huang L (2007) Thermosensitive hydrogel PEG-PLGA-PEG enhances engraftment of muscle-derived stem cells and promotes healing in diabetic wound. Mol Ther J Am Soc Gene Ther 15:1189–1194. doi:10.1038/sj.mt.6300156
Makadia HK, Siegel SJ (2011) Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 3:1377–1397. doi:10.3390/polym3031377
Nolazco G, Kovanecz I, Vernet D, Gelfand RA, Tsao J, Ferrini MG, Magee T, Rajfer J, Gonzalez-Cadavid NF (2008) Effect of muscle-derived stem cells on the restoration of corpora cavernosa smooth muscle and erectile function in the aged rat. BJU Int 101:1156–1164. doi:10.1111/j.1464-410X.2008.07507.x
Payne TR, Oshima H, Okada M, Momoi N, Tobita K, Keller BB, Peng H, Huard J (2007) A relationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts. J Am Coll Cardiol 50:1677–1684. doi:10.1016/j.jacc.2007.04.100
Qu Z, Huard J (2000) Matching host muscle and donor myoblasts for myosin heavy chain improves myoblast transfer therapy. Gene Ther 7:428–437. doi:10.1038/sj.gt.3301103
Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, Huard J (2002) Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol 157:851–864. doi:10.1083/jcb.200108150
Ren Y, Wu H, Zhou X, Wen J, Jin M, Cang M, Guo X, Wang Q, Liu D, Ma Y (2012) Isolation, expansion, and differentiation of goat adipose-derived stem cells. Res Vet Sci 93:404–411. doi:10.1016/j.rvsc.2011.08.014
Rossello RA, Kohn DH (2009) Gap junction intercellular communication: a review of a potential platform to modulate craniofacial tissue engineering. J Biomed Mater Res B Appl Biomater 88:509–518. doi:10.1002/jbm.b.31127
Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM (2008) Self-renewal and expansion of single transplanted muscle stem cells. Nature 456:502–506. doi:10.1038/nature07384
Seale P, Rudnicki MA (2000) A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev Biol 218:115–124. doi:10.1006/dbio.1999.9565
Su J, Mazzeo J, Subbarao N, Jin T (2011) Pharmaceutical development of biologics: fundamentals, challenges and recent advances. Ther Deliv 2:865–871
Tamaki T, Akatsuka A, Okada Y, Uchiyama Y, Tono K, Wada M, Hoshi A, Iwaguro H, Iwasaki H, Oyamada A, Asahara T (2008) Cardiomyocyte formation by skeletal muscle-derived multi-myogenic stem cells after transplantation into infarcted myocardium. PLoS ONE 3:e1789. doi:10.1371/journal.pone.0001789
Tisdale MJ (2002) Cachexia in cancer patients. Nat Rev Cancer 2:862–871. doi:10.1038/nrc927
Wobus AM, Boheler KR (2005) Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol Rev 85:635–678. doi:10.1152/physrev.00054.2003
Wu H, Ren Y, Li S, Wang W, Yuan J, Guo X, Liu D, Cang M (2012) In vitro culture and induced differentiation of sheep skeletal muscle satellite cells. Cell Biol Int 36:579–587. doi:10.1042/CBI20110487
Zammit PS, Heslop L, Hudon V, Rosenblatt JD, Tajbakhsh S, Buckingham ME, Beauchamp JR, Partridge TA (2002) Kinetics of myoblast proliferation show that resident satellite cells are competent to fully regenerate skeletal muscle fibers. Exp Cell Res 281:39–49
Acknowledgments
This study was supported by a grant from the Major Projects for New Varieties of Genetically Modified Organisms (2012ZX08008-002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Ren Yu and Wu Haiqing have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yu, R., Haiqing, W., Hefei, W. et al. Biological characteristics of muscle-derived satellite cells isolated from rats at different postnatal days. Cytotechnology 67, 397–408 (2015). https://doi.org/10.1007/s10616-013-9670-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-013-9670-3