Abstract
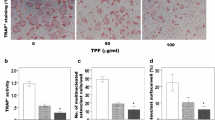
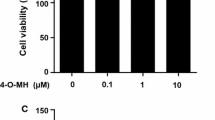
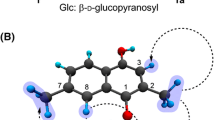
Nitensidine A is a guanidine alkaloid isolated from Pterogyne nitens, a common plant in South America. To gain insight into the biological activity of P. nitens-produced compounds, we examined herein their biological effects on osteoclasts, multinucleated giant cells that regulate bone metabolism by resorbing bone. Among four guanidine alkaloids (i.e., galegine, nitensidine A, pterogynidine, and pterogynine), nitensidine A and pterogynine exhibited anti-osteoclastic effects at 10 μM by reducing the number of osteoclasts on the culture plate whereas galegine and pterogynidine did not. The anti-osteoclastic activities of nitensidine A and pterogynine were exerted in a concentration-dependent manner, whereas nitensidine A exhibited an approximate threefold stronger effect than pterogynine (IC50 values: nitensidine A, 0.93 ± 0.024 μM; pterogynine, 2.7 ± 0.40 μM). In the present study, the anti-osteoclastic effects of two synthetic nitensidine A derivatives (nitensidine AT and AU) were also examined to gain insight into the structural features of nitensidine A that exert an anti-osteoclastic effect. The anti-osteoclastic effect of nitensidine A was greatly reduced by substituting the imino nitrogen atom in nitensidine A with sulfur or oxygen. According to the differences in chemical structures and anti-osteoclastic effects of the four guanidine alkaloids and the two synthetic nitensidine A derivatives, it is suggested that the number, binding site, and polymerization degree of isoprenyl moiety in the guanidine alkaloids and the imino nitrogen atom cooperatively contribute to their anti-osteoclastic effects.




Similar content being viewed by others
References
Bolzani VS, Gunatilaka AA, Kingston DG (1995) Bioactive guanidine alkaloids from Pterogyne nitens. J Nat Prod 58:1638–1688
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Chambers TJ (2000) Regulation of the differentiation and function of osteoclasts. J Pathol 192:4–13
Duarte RA, Mello ER, Araki C, Bolzani Vda S, Siqueira e Silva DH, Regasini LO, Silva TG, de Morais MC, Ximenes VF, Soares CP (2010) Alkaloids extracted from Pterogyne nitens induce apoptosis in malignant breast cell line. Tumour Biol 31:513–522
Edwards JR, Mundy GR (2011) Advances in osteoclast biology: old findings and new insights from mouse models. Nat Rev Rheumatol 7:235–243
Felix R, Cecchini MG, Hofstetter W, Elford PR, Stutzer A, Fleisch H (1990) Impairment of macrophage colony-stimulating factor production and lack of resident bone marrow macrophages in the osteopetrotic op/op mouse. J Bone Miner Res 5:781–789
Fernandes DC, Regasini LO, Vellosa JC, Pauletti PM, Castro-Gamboa I, Bolzani VS, Oliveira OM, Silva DH (2008) Myeloperoxidase inhibitory and radical scavenging activities of flavones from Pterogyne nitens. Chem Pharm Bull (Tokyo) 56:723–726
Fuller K, Lean JM, Bayley KE, Wani MR, Chambers TJ (2000) A role for TGFβ1 in osteoclast differentiation and survival. J Cell Sci 113:2445–2453
Kaneda T, Nojima T, Nakagawa M, Ogasawara A, Kaneko H, Sato T, Mano H, Kumegawa M, Hakeda Y (2000) Endogenous production of TGF-beta is essential for osteoclastogenesis induced by a combination of receptor activator of NF-kappa B ligand and macrophage-colony-stimulating factor. J Immunol 165:4254–4263
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Mochizuki A, Takami M, Kawawa T, Suzumoto R, Sasaki T, Shiba A, Tsukasaki H, Zhao B, Yasuhara R, Suzawa T, Miyamoto Y, Choi Y, Kamijo R (2006) Identification and characterization of the precursors committed to osteoclasts induced by TNF-related activation-induced cytokine/receptor activator of NF-kappa B ligand. J Immunol 177:4360–4368
Nakagawa H, Wachi M, Woo JT, Kato M, Kasai S, Takahashi F, Lee IS, Nagai K (2002) Fenton reaction is primarily involved in a mechanism of (−)-epigallocatechin-3-gallate to induce osteoclastic cell death. Biochem Biophys Res Commun 292:94–101
Nakagawa H, Takami M, Udagawa N, Sawae Y, Suda K, Sasaki T, Takahashi N, Wachi M, Nagai K, Woo JT (2003) Destruxins, cyclodepsipeptides, block the formation of actin rings and prominent clear zones and ruffled borders in osteoclasts. Bone 33:443–455
Nakagawa H, Hasumi K, Takami M, Aida-Hyugaji S, Woo JT, Nagai K, Ishikawa T, Wachi M (2007) Identification of two biologically crucial hydroxyl groups of (−)-epigallocatechin gallate in osteoclast culture. Biochem Pharmacol 73:34–43
Nakagawa H, Wakabayashi-Nakao K, Tamura A, Toyoda Y, Koshiba S, Ishikawa T (2009) Disruption of N-linked glycosylation enhances ubiquitin-mediated proteasomal degradation of the human ATP-binding cassette transporter ABCG2. FEBS J 276:7237–7252
Regasini LO, Vellosa JC, Silva DH, Furlan M, de Oliveira OM, Khalil NM, Brunetti IL, Young MC, Barreiro EJ, Bolzani VS (2008) Flavonols from Pterogyne nitens and their evaluation as myeloperoxidase inhibitors. Phytochemistry 69:1739–1744
Regasini LO, Castro-Gamboa I, Silva DH, Furlan M, Barreiro EJ, Ferreira PM, Pessoa C, Lotufo LV, de Moraes MO, Young MC, Bolzani Vda S (2009) Cytotoxic guanidine alkaloids from Pterogyne nitens. J Nat Prod 72:473–476
Regasini LO, Pivatto M, Scorzoni L, Benaducci T, Fusco-Almeida A, Giannini M, Barreiro EJ, Silva D, Bolzani Vda S (2010) Antimicrobial activity of Pterogyne nitens Tul., Fabaceae, against opportunistic fungi. Rev Bras Farmacogn 20:706–711
Rodan GA, Martin TJ (2000) Therapeutic approaches to bone diseases. Science 289:1508–1514
Sells Galvin RJ, Gatlin CL, Horn JW, Fuson TR (1999) TGF-beta enhances osteoclast differentiation in hematopoietic cell cultures stimulated with RANKL and M-CSF. Biochem Biophys Res Commun 265:233–239
Teitelbaum SL (2000) Bone resorption by osteoclasts. Science 289:1504–1508
Tomimori Y, Mori K, Koide M, Nakamichi Y, Ninomiya T, Udagawa N, Yasuda H (2009) Evaluation of pharmaceuticals with a novel 50-h animal model of bone loss. J Bone Miner Res 24:1194–1205
Venkatachalam TK, Qazi S, Samuel P, Uckun FM (2003) Inhibition of mast cell leukotriene release by thiourea derivatives. Bioorg Med Chem Lett 13:485–488
Wattel A, Kamel S, Mentaverri R, Lorget F, Prouillet C, Petit JP, Fardelonne P, Brazier M (2003) Potent inhibitory effect of naturally occurring flavonoids quercetin and kaempferol on in vitro osteoclastic bone resorption. Biochem Pharmacol 65:35–42
Woo JT, Nakagawa H, Notoya M, Yonezawa T, Udagawa N, Lee IS, Ohnishi M, Hagiwara H, Nagai K (2004) Quercetin suppresses bone resorption by inhibiting the differentiation and activation of osteoclasts. Biol Pharm Bull 27:504–509
Yan T, Riggs BL, Boyle WJ, Khosla S (2001) Regulation of osteoclastogenesis and RANK expression by TGF-beta1. J Cell Biochem 83:320–325
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95:3597–3602
Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S (1990) The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345:442–444
Zhao B, Takami M, Yamada A, Wang X, Koga T, Hu X, Tamura T, Ozato K, Choi Y, Ivashkiv LB, Takayanagi H, Kamijo R (2009) Interferon regulatory factor-8 regulates bone metabolism by suppressing osteoclastogenesis. Nat Med 15:1066–1071
Acknowledgments
This study was supported by a Grant-in-Aid for Young Scientists (B) (JSPS KAKENHI Grant Number 22791786) and Grant-in-Aid for Scientific Research (C) (JSPS KAKENHI Grant Number 24592822) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), and Chubu University Grant A (23IM04A) and B (23IM02B and 24M01B) from Chubu University. The isolation of the guanidine alkaloids and synthesis of the nitensidine A derivatives were supported by a grant from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) as part of the Biota-FAPESP-The Biodiversity Virtual Institute Program (www.biotasp.org.br), Grant No. 03/02176-7, awarded to V.S.B.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tajima, Y., Murase, H., Satake, K. et al. Nitensidine A, a guanidine alkaloid from Pterogyne nitens, induces osteoclastic cell death. Cytotechnology 67, 585–592 (2015). https://doi.org/10.1007/s10616-013-9590-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-013-9590-2