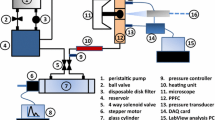
Abstract
Artery stiffening is known as an important pathological change that precedes small vessel dysfunction, but underlying cellular mechanisms are still elusive. This paper reports the development of a flow co-culture system that imposes a range of arterial-like pulse flow waves, with similar mean flow rate but varied pulsatility controlled by upstream stiffness, onto a 3-D endothelial-smooth muscle cell co-culture. Computational fluid dynamics results identified a uniform flow area critical for cell mechanobiology studies. For validation, experimentally measured flow profiles were compared to computationally simulated flow profiles, which revealed percentage difference in the maximum flow to be <10, <5, or <1% for a high, medium, or low pulse flow wave, respectively. This comparison indicated that the computational model accurately demonstrated experimental conditions. The results from endothelial expression of proinflammatory genes and from determination of proliferating smooth muscle cell percentage both showed that cell activities did not vary within the identified uniform flow region, but were upregulated by high pulse flow compared to steady flow. The flow system developed and characterized here provides an important tool to enhance the understanding of vascular cell remodeling under flow environments regulated by stiffening.











Similar content being viewed by others
References
Berger RMF, Cromme-Dijkhuis AH, Hop CJ, Kruit MN, Hess J (2002) Pulmonary arterial wall distensibility assessed by intravascular ultrasound in children with “congenital heart disease an indicator for pulmonary vascular disease?”. Chest 122:549–557
Bjork TR (2009) Transmural flow bioreactor for vascular tissue engineering. Biotechnol Bioeng 104:1197–1206
Chesler NC, Thompson-Figueroa J, Millburne K (2004) Measurements of mouse pulmonary artery biomechanics. J Biomech Eng 126:309–314
Cheung N, Sharrett AR, Klein R, Criqui MH, Islam FM, Macura KJ, Cotch MF, Klein BE, Wong TY (2007) Aortic distensibility and retinal arteriolar narrowing: the multiethnic study of atherosclerosis. Hypertension 50:617–622
Chien S (2007) Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol 292:H1209–H1224
Chiu JJ, Chen LJ, Lee PL, Lee CI, Lo LW, Usami S, Chein S (2003) Shear stress inhibits adhesion molecule expression in vascular endothelial cells induced by coculture with smooth muscle cells. Blood 101:2667–2674
Chung BJ, Roberston AM, Peters DG (2003) The numerical design of a parallel plate flow chamber for investigation of endothelial cell response to shear stress. Comput Struct 81:535–546
Chung CC, Chen CP, Tseng CS (2007) Enhancement of cell growth in tissue-engineering constructs under direct perfusion: modeling and simulation. Biotechnol Bioeng 97:1603–1616
Cotter S (1979) A screening design for factorial experiments with interactions. Biometrika 66:317–320
Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA (2004) Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and resistant regions of human vasculature. Proc. Natl. Acad. Sci. USA 101:14871–14876
Davies PF (2009) Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6:16–26
Fillinger MF, Sampson LN, Cornenwett JL, Powell RJ, Wagner RJ (1997) Coculture of endothelial cells and smooth muscle cells in bilayer and conditioned media models. J Surg Res 67:169–178
Gimbrone MA, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G (2006) Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann NY Acad Sci 902:230–240
Gorgulu S, Eren M, Yildirim A, Ozer O, Uslu N, Celik S, Dagdeviren B, Nurkalem Z, Bagirtan B, Tezel T (2003) A new echocardiographic approach in assessing pulmonary vascular bed in patients with congenital heart disease: pulmonary artery stiffness. Anadolu Kardiyol Derg 3:92–97
Hahn C, Schwartz MA (2009) Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol 10:53–62
Hastings NE, Simmers MB, McDonald OG, Wamhoff BR, Blackman BR (2007) Atherosclerosisprone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes proinflammatory priming. Am J Physiol Cell Physiol 293:1824–1833
Heydarkhan-Hagvall S, Helenius G, Johanasson BR, Li JY, Mattsson E, Risberg B (2003) Co-culture of endothelial cells and smooth muscle cells affect gene expression of angiogenic factors. J Cell Biochem 89:1250–1259
Hsiai TK, Cho SK, Honda HM, Hama S, Navab M, Demer LL, Ho CM (2002) Endothelial cell dynamics under pulsating flows: significance of high verse low shear stress slew rates. Ann Biomed Eng 30:646–656
Hsiai TK, Cho SK, Wong PK, Ing M, Salazar A, Sevanian A, Navab M, Demer LL, Ho CM (2003) Monocyte recruitment to endothelial cells in response to oscillatory shear stress. FASEB J 17:1648–1657
Hunter K, Lee P, Lanning C, Ivy D, Kirby K, Claussen L, Chan K, Shandas R (2008) Pulmonary vascular input impedance is a combined measure of pulmonary vascular resistance and stiffness and predicts clinical outcomes better than pulmonary vascular resistance alone in pediatric patients with pulmonary hypertension. Am Heart J 155:166–174
Li M, Scott D, Shandas R, Stenmark KR, Tan W (2009) High pulsatility flow induces adhesion molecule and cytokine mRNA Expression in distal pulmonary artery endothelial cells. Ann Biomed Eng 37:1082–1092
McVeigh GE, Bratteli CW, Morgan DJ, Alinder CM, Glasser SP, Finkelstein SM, Cohn JN (1999) Age-related abnormalities in arterial compliance identified by pressure pulse contour analysis. Hypertension 33:1392–1398
Mitchell GF (2008) Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol 105:1652–1660
Nauman EA, Risic KJ, Keaveny TM, Satcher RL (1999) Quantitative assessment of steady and pulsatile flow fields in parallel plate flow chamber. Ann Biomed Eng 27:194–199
O’Rourke MF, Safar ME (2005) Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 46:200–204
Panaritis V, Kyriakidis AV, Pyrgioti M, Raffo L, Anagnostopoulou E, Gourniezaki G, Koukou E (2005) Pulsatility index of temporal and renal arteries as an early finding of arteriopathy in diabetic patients. Ann Vas Sur 19:80–83
Reneman RS, Arts T, Hoeks APG (2006) Wall shear stress—an important determinant of endothelial cell function and structure—in the arterial system in vivo. J Vasc Res 43:251–269
Safar ME, Lacolley P (2007) Disturbance of maro- and microcirculations: relations with pulse pressure and cardiac organ damage. Am J Physiol Heart Circ Physiol 293:H1–H7
Safar ME, Levy BI, Struijker-Boudier H (2003) Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 107:2864–2869
Sakao S, Tatsumi K, Voelkel NF (2009) Endothelial cells and pulmonary arterial hypertension: apoptosis, proliferation, interaction and transdifferentiation. Respir Res 10:95
Shi Y (2008) Numerical simulation of global hydro-dynamics in a pulsatile bioreactor for cardiovascular tissue engineering. J Biomech 41:953–959
Shyy YJ, Hsieh HJ, Usami S, Chien S (1994) Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proc. Natl. Acad. Sci. USA 91:4678–4682
Susilo M, Roeder BA, Voytik-Harbin SL, Lokini K, Nauman EA (2010) Development of a three dimensional unit cell to model the micromechanical response of a collagen-based extracellular matrix. Acta Biomater 6:1471–1486
Tada A, Tarbell JM (2000) Interstitial flow through the internal elastic lamina affects shear stress on arterial smooth muscle cells. Am J Physiol Heart Circ Physiol 278:H1589–H1597
Tsubio H, Ando J, Korenaga R, Takada Y, Kamiya A (2006) Flow stimulates ICAM-1 expression time and shear stress dependently in cultured human endothelial cells. Biochem Biophy Res Comm 6:988–996
Undar A, Zapanta CM, Reibson JD, Souba M, Lukic B, Weiss WJ, Snyder AJ, Kunselman AR, Peirce WS, Rosenberg G, Myers JL (2005) Precise quantification of pressure flow waveforms of a pulsatile ventricular assist device. ASAIO 51:56–59
Walshe TE, Ferguson G, Connell P, O’Brien C, Cahill PA (2005) Pulsatile flow increases the expression of eNOS, ET-1, and prostacyclin in a novel in vitro coculture model of the retinal vasculature. Invest Ophthalmol Vis Sci 46:375–382
Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Berent R, Eber B (2004) Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation 109:184–189
Yee A, Bosworth KA, Conway DE, Eskin SG, McIntire LV (2008) Gene expression of endothelial cells under pulsatile non-reversing vs. steady shear stress; comparison of nitric oxide production. Ann Biomed Eng 36:571–579
Acknowledgments
The authors wish to thank CVP group for providing the cells and acknowledge funding from American Heart Association (SDG 2110049 to W.T.), and NIH (HL K25 097246 to W.T., HL T32 072738 to R.S.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Scott-Drechsel, D., Su, Z., Hunter, K. et al. A new flow co-culture system for studying mechanobiology effects of pulse flow waves. Cytotechnology 64, 649–666 (2012). https://doi.org/10.1007/s10616-012-9445-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-012-9445-2