Abstract
The production of therapeutic proteins requires qualification of equipment components and appropriate validation procedures for all operations. Since protein productions are typically performed in bioreactors using aerobic cultivation processes air sparging is an essential factor. As recorded in literature, besides ring spargers and open pipe, sinter frits are often used as sparging elements in large scale bioreactors. Due to the manufacturing process these frits have a high lot-to-lot product variability. Experience shows this is a practical problem for use in production processes of therapeutic proteins, hence frits must be tested before they can be employed. The circumstance of checking quality and performance of frits as sparging elements was investigated and various possibilities have been compared. Criteria have been developed in order to evaluate the sparging performance under conditions comparable to those in production bioreactors. The oxygen mass transfer coefficient (k L a) was chosen as the evaluation criterion. It is well known as an essential performance measure for fermenters in the monoclonal antibody production. Therefore a test rig was constructed able to automatically test frit-spargers with respect to their k L a-values at various gas throughputs. Performance differences in the percent range could be detected.









Similar content being viewed by others
References
Bohlender (2010) Teflonfrits, Internet presentation, Bohlender GmbH—Waltersberg 8—D-97947 Grünsfeld—Germany, e-mail: info@bola.de
Cussler EL (2008) Diffusion and mass transfer in fluid systems, Second Edition, 9th printing, Cambridge University Press, ISBN: 978-0-521-56477-9, p 112
GKN (2010) Edelstahlfritten, GKN Sinter Metals Filters GmbH, Dahlienstrasse 43, D-42477 Radevormwald, e-mail: info@gkn-filters.com, www.gkn-filters.com
Gray DR, Chen S, Howarth W, Inlow D, Maiorella BL (1996) CO2 in large-scale and high-density CHO cell perfusion culture. Cytotechnology 22:65–78
Nienow AW (2006) Reactor engineering in large scale animal cell culture. Cytotechnology 50:9–33
Ozturk SS (1996) Engineering challenges in high density cell culture systems. Cytotechnology 22:3–16
ROBU (2010) Al2O3-Frits, ROBU Glasfilter-Geräte GmbH, Schützenstrasse 13, D-57644 Hattert
Wlaschin KF, Hu WS (2006) Fedbatch culture and dynamic nutrient feeding. Adv Biochem Eng Biotechnol 101:43–74
Acknowledgments
The help of K. Ostmann during the measurements is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
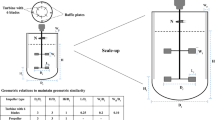
See Table 1.
Rights and permissions
About this article
Cite this article
Sieblist, C., Aehle, M., Pohlscheidt, M. et al. A test facility for fritted spargers of production-scale-bioreactors. Cytotechnology 63, 49–55 (2011). https://doi.org/10.1007/s10616-010-9325-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-010-9325-6