Abstract
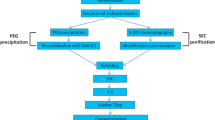
To establish a cost-effective purification process for the large-scale production of plasmid DNA for gene therapy and DNA vaccination, a single anion-exchange chromatography (AEC) step was employed to purify supercoiled plasmid DNA (sc pDNA) from other isoforms and Escherichia coli impurities present in a clarified lysate. Two different size and conformation plasmids were used as model targets, and showed similar elution behavior in this chromatographic operation, in which sc pDNA was effectively separated from open circle plasmid DNA (oc pDNA) in a salt gradient. The process delivered high-purity pDNA of homogeneity of 95 ± 1.1% and almost undetectable levels of endotoxins, genomic DNA, RNA and protein, at a yield of 65 ± 8%. Furthermore, the transfection efficiency (29 ± 0.4%) was significantly higher than that (20 ± 0.1%) of a pDNA control. The present study confirms the possibility of using a single AEC step to purify sc pDNA from other isoforms and host contaminants present in a clarified E. coli lysate.




Similar content being viewed by others
References
Bencina M, Podgornik A, Strancar A (2004) Characterization of methacrylate monoliths for purification of DNA molecules. J Sep Sci 27:801–810
Cupillard L, Juillard V, Latour S, Colombet G, Cachet N, Richard S et al (2005) Impact of plasmid supercoiling on the efficacy of a rabies DNA vaccine to protect cats. Vaccine 23:1910–1916
Diogo MM, Queiroz JA, Monteiro GA, Martins SA, Ferreira GN, Prazeres DMF (2000) Purification of a cystic fibrosis plasmid vector for gene therapy using hydrophobic interaction chromatography. Biotechnol Bioeng 68:576–583
Diogo MM, Ribeiro SC, Queiroz JA, Monteiro GA, Tordo N, Perrin P et al (2001) Production, purification and analysis of an experimental DNA vaccine against rabies. J Gene Med 3:577–584
Diogo MM, Queiroz JA, Prazers DMF (2003) Assessment of purity and quantification of plasmid DNA in process solutions using high-performance hydrophobic interaction chromatography. J Chromatogr A 998:109–117
Diogo MM, Queiroz JA, Prazeres DMF (2005) Chromatography of plasmid DNA. J Chromatogr A 1069:3–22
Ferreira GNM, Cabral JMS, Prazeres DMF (1997) A comparison of gel filtration chromatographic supports for plasmid purification. Biotechnol Tech 11:417–420
Gurunathan S, Klinman DM, Seder RA (2000) DNA vaccines: immunology, application, and optimization. Ann Rev Immunol 18:927–974
Hines RN, O’Connor KC, Vella G, Warren W (1992) Large-scale purification of plasmid DNA by anion-exchange high-performance liquid chromatography. Biotechniques 12:430–434
Horn NA, Meek JA, Budahazi G, Marquet M (1995) Cancer gene therapy using plasmid DNA: purification of DNA for human clinical trials. Hum Gene Ther 6:565–573
Iuliano S, Fisher JR, Chen M, Kelly WJ (2002) Rapid analysis of a plasmid by hydrophonic-interaction chromatography with a non-porous resin. J Chromatogr A 972:77–86
Pillai VB, Hellerstein M, Yu T, Amara RR, Robinson HL (2008) Comparative studies on in vitro expression and in vivo immunogenicity of supercoiled and open circular forms of plasmid DNA vaccines. Vaccine 26:1136–1141
Prazeres DMF, Schluep T, Cooney C (1998) Preparative purification of supercoiled plasmid DNA using anion-exchange chromatography. J Chromatogr A 806:31–45
Sandberg LM, Bjurling A, Busson P, Vasi J, Lemmens R (2004) Thiophilic interaction chromatography for supercoiled plasmid DNA purification. J Biotech 109:193–199
Schleef M, Schmidt T (2004) Animal-free production of ccc-supercoiled plasmids for research and clinical applications. J Gene Med 6:S45–S53
Schluep T, Cooney CL (1998) Purification of plasmids by triplex affinity interaction. Nucleic Acids Res 26:4524–4528
Wils P, Escriou V, Warnery A, Lacroix F, Lagneaux D, Ollivier M et al (1997) Efficient purification of plasmid DNA for gene transfer using triple-helix affinity chromatography. Gene Ther 4:323–330
Acknowledgments
Supported by National Natural Science Foundation (30572124), Guangdong Natural Science Foundation (5002855), Guangdong Science and Technology Plan Project (2004B31201001) and Guangdong Pharmaceutical University doctor science starting foundation
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Bo, H., Wang, J. et al. Separation of supercoiled from open circular forms of plasmid DNA, and biological activity detection. Cytotechnology 63, 7–12 (2011). https://doi.org/10.1007/s10616-010-9322-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-010-9322-9