Abstract
A turbot, Scophthalmus maximus, fin (TF) cell line was established and susceptibility to turbot reddish body iridovirus (TRBIV) was determined in this study. Primary culture of TF cells was initiated from fin tissue pieces partially digested with trypsin, collagenase II and hyaluronidase. Digested tissue pieces were cultured at 24 °C in Leibovitz-15 medium (pH 7.2), supplemented with 20% fetal bovine serum, carboxymethyl chitosan, N-acetylglucosamine hydrochloride, basic fibroblast growth factor and epidermal growth factor. The cultured TF cells, in fibroblast shape, proliferated to 100% confluency 50 days later. A TF cell line, with a population doubling time of 45.6 h at passage 80, has been established and subcultured to passage 133. Chromosome analyses indicated that the TF cells exhibited chromosomal aneuploidy with a modal chromosome number of 44 which displayed the normal diploid karyotype of S. maximus at least up to passage 80. TRBIV susceptibility testing demonstrated that cytopathic effect and propagated viral particles were observed in TF cells after TRBIV infection. In conclusion, a continuous TRBIV susceptible TF cell line has been established successfully, and the cell line may serve as a valuable tool for studies of cell-virus interactions and has applications for different kinds of cytotechnological studies as well.
Similar content being viewed by others
Introduction
Turbot, Scophthalmus maximus, is a kind of important commercial flatfish which has been intensively cultured in Spain and north China (Lei et al. 2005). The decline of the turbot fishery has been accompanied by environment pollution, expanded farming and increased occurrence of infective diseases (Shi et al. 2003). A new type of iridovirus, turbot reddish body iridovirus (TRBIV) (Megalocytivirus, Iridoviridae), has been identified as the primary cause of viral reddish body syndrome in turbot aquaculture, associated with high transmissibility and mortality (Shi et al. 2004, 2005). Increased knowledge of the viral pathogens and associated diseases of turbot will be necessary to manage turbot diseases and contribute to large-scale aquaculture success. Since cell lines are ideal tools for in vitro studies of cell-virus interactions, virus propagation, isolation and vaccine development (Villena 2003), it is important to develop specific cell lines from the species being cultured, in this case turbot S. maximus, for viral disease investigations. Many fish cell lines have been established but consist primarily of freshwater and anadromous species (Fryer and Lannan 1994; Villena 2003). Only few cell lines have been established from commercial marine fishes, such as sea perch (Lateolabrax japonicus) (Ye et al. 2006; Chen et al. 2007), turbot (S. maximus) (Fernández-Puentes et al. 1993; Fernández-Puentes and Figueras 1996; Chen et al. 2005), flounder (Paralichthys olivaceus) (Tong et al. 1997; Chen et al. 2004), red sea bream (Pagrus major) (Zhou et al. 2003; Imajoh et al. 2007), seabass (Lates calcarifer) (Lakra et al. 2006) and groupers (Lai et al. 2000, 2003; Qin et al. 2006; Parameswaran et al. 2007; Zhou et al. 2007; Wen et al. 2008; Wei et al. 2009). Among the three turbot cell lines established, no TRBIV susceptibility of them has been reported (Fernández-Puentes et al. 1993; Fernández-Puentes and Figueras 1996; Chen et al. 2005). Currently, only two marine fish cell lines have been found to have susceptibilities to TRBIV (Fan et al. 2006; Wei et al. 2009). By now, no continuous turbot fin cell lines with TRBIV susceptibilities have been established from turbot S. maximus. This study was intended to establish a turbot fin cell line and to characterize its susceptibility to TRBIV.
Materials and methods
Animals
Healthy turbot Scophthalmus maximus (about 10–15 cm in length), were obtained from Hesheng turbot farm (Laizhou, Shandong, China). They were maintained in aerated sterile seawater containing 1,000 IU/mL penicillin and 1,000 μg/mL streptomycin at 22–24 °C for 24 h.
In vitro culture
After euthanasia by etherification, turbots were immersed in 70% ethanol for 1–2 min. The fin tissues were removed aseptically, washed once with 70% alcohol and twice with phosphate-buffered saline (PBS), and minced into small pieces (about 1 mm3 in size) in Leibovitz L-15 medium (Invitrogen)(pH 7.2) containing 5% fetal bovine serum (FBS)(Thermo Fisher Scientific) by surgical scissors. After digestion with 0.25% trypsin for 20 min, the fin tissue pieces were digested with 0.5% hyaluronidase and 0.2% collagenase II (Solarbio) for 120 min. The partially digested tissue pieces were centrifuged at 120 g for 10 min, and pellets resuspended in 5% FBS-containing L-15 medium and inoculated into 25 cm2 cell culture flasks (Corning)(1 mL in each flask). After cultured at 24 °C for about 12 h, 5 mL of 20% FBS-containing L-15 medium supplemented with 100 μg/mL carboxymethyl-chitosan (AK Scientific), 50 μg/mL N-acetylglucosamine hydrochloride (Sigma-Aldrich), 10 ng/mL basic fibroblast growth factor (bFGF) (Peprotech), 40 ng/mL insulin-like growth factor-I (IGF-I) (Peprotech), 100 IU/mL penicillin and 100 μg/mL streptomycin (Lukang) were added to each flask. These flasks were incubated at 24 °C and the medium was replaced every 5 days.
Once turbot fin (TF) cells grew into a confluent monolayer, the cells were subcultured by trypsinisation as described previously (Wei et al. 2009). From passage 30, the culture medium was changed from 20% FBS-containing L-15 medium into 20% bovine calf serum (BCS)(Thermo Fisher Scientific)-containing Minimum Essential Medium (MEM)(Invitrogen) without any supplements as described above.
Growth properties
TF cells at passage 80 were trypsinised and resuspended in 20% BCS-containing MEM medium as described above. About 1 mL of TF cell suspension with a density of 2.7 × 105 cells/mL was dispensed into each well of two 24-well plates (Corning) and incubated at 24 °C in a 5% CO2 incubator (Heal Force HF240). Three wells of TF cells were harvested by trypsinisation and resuspended in 1 mL PBS at 12 h intervals. The number of cells in each well was counted by a cell analysis system (CASY), and the average value of 3 wells at each time was used to plot the growth curve. The population doubling time of TF cells was calculated according to the method described preciously (Fan et al. 2007).
Chromosome analysis
The passage 80 TF cells at the logarithmic phase were treated with 20 μg/mL of colchicine (Shanghai Chemical Reagents) for 10 h at 24 °C. The cells were harvested by trypsinisation and resuspended in 0.3% KCl hypotonic solution, fixed with Carnoy’s solution and stained with Giemsa for 40–50 min. The chromosome analysis of TF cells was performed according to the method described preciously (Fan et al. 2007), and chromosome numbers of at least 200 metaphase TF cells were counted by a Nikon E200 light microscope.
Viral susceptibility
The viral susceptibilities of passage 80 TF cells to turbot reddish-body iridovirus (TRBIV) was evaluated according to methods described previously (Fan et al. 2006). TRBIV had been isolated and identified from TRBIV-infected turbot previously and stored at −86 °C (Fan et al. 2006). After removal of growth medium, TF cells in the logarithmic growth phase were washed twice with PBS and inoculated with 0.2 mL TRBIV suspension for 2 h at a multiplicity of infection (MOI) of 0.01. The TRBIV suspension was removed and the fin cells were incubated in 10% FBS-containing MEM medium at 24 °C. Cytopathic effect (CPE) was observed and recorded daily utilizing a Nikon TS100 inverted light microscope.
Electron microscopy of virus-infected TF cells
After CPE appeared, TRBIV-infected TF cells were collected by 0.25% trypsin digestion (1–2 min) and centrifugation (120 g, 10 min). The TF cells were fixed with 2.5% glutaraldehyde in 0.2 M sodium cacodylate buffer (pH 7.4) for 8 h at 4 °C. After washing with sodium cacodylate buffer and postfixing with 1% osmium tetroxide for 1.5 h, the fixed cells were dehydrated and embedded in epoxy resin. Ultrathin sections were stained with 2% uranyl acetate-lead citrate and observed by a transmission electron microscope (JEOL JEM1200EX).
Results
In vitro culture of TF cells
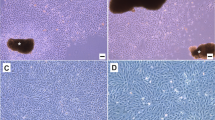
The TF cells from S. maximus in primary culture had fibroblastic morphology and proliferated to 100% confluence 50 days later (Fig. 1a, b). The cells grew in 20% FBS-containing L-15 medium supplemented with carboxymethyl-chito-oligosaccharide, N-acetylglucosamine hydrochloride, bFGF and IGF-I, and could be subcultured at 5–7 day intervals (Fig. 1c). The TF cells grew at a steady rate and their doubling time was calculated to be 45.6 h at passage 80 (Fig. 2). The TF cells have been subcultured to passage 133 (Fig. 1d), and a continuous TF cell line has been established.
In vitro cultured turbot fin (TF) cells. a TF cells migrated from fin tissues 48 h after primary culture initiation. b the confluent monolayer formed by the TF cells 50 days later, showing the fibroblastic morphology of TF cells. c subcultured TF cells at passage 80. d subcultured TF cells at passage 133
Chromosome analysis of TF cells
The results of chromosome counts of 197 metaphase TF cells at passage 80 revealed that the chromosome numbers varied from 22 to 88 with a modal chromosome number of 44, which accounted for 64.47% of the metaphase cells (Fig. 3a). The distribution was asymmetrical and both aneuploidy and heteroploidy appeared in the TF cell line. The metaphase chromosomes (Fig. 3b) with a normal diploid number of 44 displayed the normal karyotype morphology, consisting of 2 pairs of metacentrics (m), 6 pairs of submetacentrics (sm), 5 pairs of subtelocentrics (st), and 9 pairs of telocentrics (t): 2n = 4m + 2sm + 10st + 28t, NF = 60 (Fig. 3c).
Chromosome analyses of turbot fin (TF) cells at passage 80. a the chromosomal aneuploidy of TF cells with chromosome numbers ranged from 22 to 88. About 64.47% of TF cells had a chromosome number of 44. b chromosomes from a TF cell with a number of 44. c the diploid karyotype of TF cells, 2n = 44, 4m + 2sm + 10st + 28t, NF = 60
Cell susceptibility to TRBIV
Following inoculation with TRBIV, CPE was observed in TF cells within 2 days (Fig. 4b). As CPE development progressed, cells became round and wizened with cell aggregation. Additional adjoining cells became granular and vacuolated in 3–4 days (Fig. 4c, d). Besides, the culture supernatant of TRBIV-infected TF cells could reinfect other normal TF cells. The viral titers of TRBIV in TF cells reached 104.3 TCID50/mL within 4 days.
Viral susceptibility of TRBIV-infected turbot fin (TF) cells. a normal TF cells without any virus infection. b TF cells infected by TRBIV at day 2, early formed CPE is evident. c TF cells infected by TRBIV at day 3, showing typical CPE of TRBIV in TF cells. d TF cells infected by TRBIV at day 4. Round and condensed morphology of TF cells can be seen
Viral particles in TRBIV-infected TF Cells
Electron microscopy observations showed many viral particles accumulated in the cytoplasm of TRBIV-infected TF cells (Fig. 5a). In ultrathin sections, these virions were hexagonal and enveloped (Fig. 5b), which showed typical characteristics of TRBIV (Shi et al. 2004; Wei et al. 2009).
Discussion
For studies of cell-virus interactions and turbot virus propagation, a TF cell line from turbot S. maximus was established. This cell line has been subcultured to passage 133 and viral susceptibility testing demonstrated that the TF cells could be a valuable tool for TRBIV disease investigation.
To initiate the primary culture of TF cells, turbot fin tissues were digested with hyaluronidase and collagenase II to lyse and loosen the extracellular matrix for fin cells to migrate out much more easily from tissue fragments. After digestion, the TF cells migrated out much rapidly from tissue fragments, and grew very well at 24 °C in 20% FBS-containing L-15 medium (pH 7.2). Similar methods have been reported in the establishment of brown-marbled grouper, (E. fuscoguttatus) fin cell line (Wei et al. 2009), and primary culture of skate (Raja porasa)(Fan et al. 2003) and shark (Heterodortus japonicus)(Yu et al. 2005) cartilage cells.
To induce in vitro cell proliferation, attempts have been made to replenish the culture medium with different supplements (Fan and Wang 2002; Fan et al. 2003; Yu et al. 2005; Jin et al. 2008; Wei et al. 2009). Among them, carboxymethyl chitosan (a kind of chitosan derivative) and N-acetylglucosamine hydrochloride (an acetyl form of glucosamine hydrochloride) were both found to have a positive effect on cell attachment and growth (Fan and Wang 2002). Growth factors such as bFGF and EGF have important regulatory abilities in cell proliferation, migration and differentiation. They probably activate tyrosine kinase by binding to tyrosine kinase receptor and speed cell proliferation via ras, MAPK and/or protein kinase C pathway (Boonstra et al. 1995; Hrzenjak and Shain 1995). Similar effects of the supplements on acceleration of cell attachment and growth were reported in the establishment of some marine fish cell lines and their primary cultures (Iida et al. 1998; Fan et al. 2003; Yu et al. 2005; Chen et al. 2007; Wei et al. 2009). Addition of all these supplements in the culture medium is most probably the key premise of inducing cell proliferation in primary culture and successful subculture of TF cells in this study. The cells of the established TF cell line proliferated actively during subculture and have a population doubling time of 45.6 h at passage 80.
Chromosome analyses demonstrated that the TF cells at passage 80, exhibited chromosomal aneuploidy, still had a modal chromosome number of 44. The karyotype of 2n = 4m + 2sm + 10st + 28t (NF = 60) of the TF cells was identical to that of S. maximus (Bouza et al. 1994), and similar to that of S. maximus (2n = 4m + 12st + 28t, NF = 60) (Chen et al. 2005) in which 2 submetacentric chromosomes might be misdistinguished as subtelocentric chromosomes.
Fish iridoviruses, such as TRBIV, are significant pathogens that have been linked to mortalities of turbots (Shi et al. 2003, 2004, 2005). The application of the TF cell line for TRBIV detection was determined by cell susceptibility tests. It was found that the TF cell line exhibited excellent susceptibility to TRBIV with CPE appeared at day 2 and viral titers of 104.3 TCID50/mL following infection. In ultrathin sections, TRBIV virions, proliferated in the cytoplasm of infected TF cells, were found to be hexagonal and enveloped which was similar to the typical characteristics of TRBIV virions reported previously (Shi et al. 2004; Wei et al. 2009). Recently, only two marine fish cell lines, and a grouper fin cell line, have been reported to be available for TRBIV isolation and propagation (Fan et al. 2006; Wei et al. 2009). The susceptibility of the TF cell line to TRBIV is slightly higher than that of the grouper fin cell line (bmGF-1) whose CPE appeared at day 2 and produced viral titers of 103.2 TCID50/mL following infection (Wei et al. 2009), and higher than that of the flounder gill cell line (FG9307) whose CPE appeared at day 2 and produced viral titers of 102.8 TCID50/mL following infection (Tong and Miao 1997; Fan et al. 2006). The TF cell line established in this study has excellent susceptibility to TRBIV which will provide a valuable tool to propagate TRBIV in vitro for viral vaccine development.
In conclusion, a continuous TF cell line, with excellent susceptibility to TRBIV, has been successfully established from turbot S. maximus, and can be used as an important in vitro tool for TRBIV propagation, vaccine development, cell-virus interation studies and different kinds of applicable cytotechnological studies as well.
References
Boonstra J, Rijken P, Humbel B, Cremers F, Verkleij A, van Bergen en Henegouwen P (1995) The epidermal growth factor. Cell Biol Int 19:413–430
Bouza C, Sanchez L, Martfnez R (1994) Karotypic characterization of turbot (Scophthalmus maximus) with conventional, fluorochrome and restriction endonucleasebanding techniques. Mar Biol 120:609–613
Chen SL, Ren GC, Sha ZX, Shi CY (2004) Establishment of a continuous embryonic cell line from Japanese flounder Paralichthys olivaceus for virus isolation. Dis Aquat Organ 60:241–246
Chen SL, Ren GC, Sha ZX, Hong YH (2005) Development and characterization of a continuous embryonic cell line from turbot (Scophthalmus maximus). Aquaculture 249:63–68
Chen SL, Sha ZX, Ye HQ, Liu Y, Tian YS, Hong Y et al (2007) Pluripotency and chimera competence of an embryonic stem cell line from the sea perch (Lateolabrax japonicus). Mar Biotechnol (NY) 9:82–91
Fan TJ, Wang XF (2002) In vitro culture of embryonic cells from shrimp Penaeus chinensis. J Exp Mar Bio Ecol 267:175–184
Fan TJ, Jin LY, Wang XF (2003) Initiation of cartilage cell culture from skate (Raja porasa Günther). Mar Biotechnol (NY) 5:64–69
Fan TJ, Wang LY, Geng XF, Cong RS, Li MY, Yu QT et al (2006) Studies of the propagation of turbot reddish body iridovirus in vitro. Period Ocean Univ China 36:767–774
Fan T, Zhao J, Fu Y, Cong R, Guo R, Liu W et al (2007) Establishment of a novel corneal endothelial cell line from domestic rabbit, Oryctolagus curiculus. Sci China C Life Sci 50:161–169
Fernández-Puentes C, Figueras A (1996) Epithelial cell line from turbot (Scophthalmus maximus L). In Vitro Cell Dev Biol Anim 32:391–393
Fernández-Puentes C, Novoa B, Figueras A (1993) Initiation of a cell line from turbot (Scophthalmus maximus L). In Vitro Cell Dev Biol Anim 29a:899–900
Fryer JL, Lannan CN (1994) Three decades of fish cell culture: A current listing of cell lines derived from fishes. J Tissue Cult Methods 16:87–94
Hrzenjak M, Shain SA (1995) Protein kinase C-dependent and -independent pathways of signal transduction in prostate cancer cells: fibroblast growth factor utilization of a protein kinase C-independent pathway. Cell Growth Differ 6:1129–1142
Iida J, Meijne AM, Oegema TR Jr, Yednock TA, Kovach NL, Furcht LT et al (1998) A role of chondroitin sulfate glycosaminoglycan binding site in alpha4beta1 integrin mediated melanoma cell adhesion. J Biol Chem 273:5955–5962
Imajoh M, Ikawa T, Oshima S (2007) Characterization of a new fibroblast cell line from a tail fin of red sea bream, Pagrus major, and phylogenetic relationships of a recent RSIV isolate in Japan. Virus Res 126:45–52
Jin LM, Wei CZ, Tian WJ, Zhao XJ, Wang YL, Fan SD (2008) Chitooligosaccharide and its derivatives promote the proliferation of osteoblasts in vitro. J Clin Rehabil Tissue Eng Res 12:3637–3640
Lai YS, Murali S, Ju HY, Wu MF, Guo IC, Chen SC et al (2000) Two iridovirus-susceptible cell lines established from kidney and liver of grouper, Epinephelus awoara (Temminck & Schlegel), and partial characterization of grouper iridovirus. J Fish Dis 23:379–388
Lai YS, John JA, Lin CH, Guo IC, Chen SC, Fang K, Lin CH, Chang CY (2003) Establishment of cell lines from a tropical grouper, Epinephelus awoara (Temminck & Schlegel), and their susceptibility to grouper irido- and nodaviruses. J Fish Dis 26:31–42
Lakra WS, Sivakumar N, Goswami M, Bhonde RR (2006) Development of two cell culture systems from Asian seabass Lates calcarifer (Bloch). Aquac Res 37:18–24
Lei J, Ma A, Chen C, Zhuang Z (2005) The present status and sustainable development of turbot (Scophthalmus maximus L) culture in China. Eng Sci (CHN) 7(5):30–35
Parameswaran V, Ishaq Ahmed VP, Shukla R, Bhonde RR, Sahul Hameed AS (2007) Development and characterization of two new cell lines from milkfish (Chanos chanos) and grouper (Epinephelus coioides) for virus isolation. Mar Biotechnol (NY) 9:281–291
Qin QW, Wu TH, Jia TL, Hegde A, Zhang RQ (2006) Development and characterization of a new tropical marine fish cell line from grouper, Epinephelus coioides susceptible to iridovirus and nodavirus. J Virol Methods 131:58–64
Shi CY, Wang YG, Huang J, Wang QY (2003) The progress on viral diseases research of turbot Scophthalmus maximus. High Biotechnol Lett (CHN) 9:99–105
Shi CY, Wang YG, Yang SL, Huang J, Wang QY (2004) The first report of an iridovirus-like agent infection in farmed turbot Scophthalmus maximus in China. Aquaculture 236:11–25
Shi CY, Wang YG, Qin L, Zhang Z, Yang B, Yang SL (2005) Investigation of pathogen and epidemiology of a novel disease, ‘Viral reddish body syndrome’, among farmed Scophthalmus maximus in China. Mar Fish Res (CHN) 26:1–6
Tong SL, Li H, Miao HZ (1997) The establishment and partial characterization of a continuous fish cell line FG-9307 from the gill of flounder Paralichthys olivaceus. Aquaculture 156:327–333
Villena AJ (2003) Applications and needs of fish and shellfish cell culture for disease control. Rev Fish Biol Fish 13:111–140
Wei YB, Fan TJ, Jiang GJ, Sun A, Xu XH, Wang J (2009) Establishment of a novel fin cell line from Brown-marbled grouper, Epinephelus fuscoguttatus (Forsskål), and evaluation of its viral susceptibility. Aquac Res 40:1523–1531
Wen CM, Lee CW, Wang CS, Cheng YH, Huang HY (2008) Development of two cell lines from Epinephelus coioides brain tissue for characterization of betanodavirus and megalocytivirus infectivity and propagation. Aquaculture 278:14–21
Ye HQ, Chen SL, Sha ZX, Xu MY (2006) Development and characterization of cell lines from heart, liver, spleen and head kidney of sea perch Lateolabrax japonicus. J Fish Biol (Suppl A) 69:115–126
Yu QT, Fan TJ, Wang XF, Cong RS, Tang ZH, Lü CX et al (2005) In vitro culture of cartilage cells from shark, Heterodortus japonicus. Mar Sci (CHN) 3:33–37
Zhou LR, Deane EE, Woo NYS (2003) Development of a black sea bream fibroblast cell line and its potential use as an in vitro model for stress protein studies. Fish Physiol Biochem 29:255–262
Zhou GZ, Li ZQ, Yuan XP, Zhang QY (2007) Establishment, characterization, and virus susceptibility of a new marine cell line from red spotted grouper (Epinephelus akaara). Mar Biotechnol (NY) 9:370–376
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, TJ., Ren, BX., Geng, XF. et al. Establishment of a turbot fin cell line and its susceptibility to turbot reddish body iridovirus. Cytotechnology 62, 217–223 (2010). https://doi.org/10.1007/s10616-010-9281-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-010-9281-1