Abstract
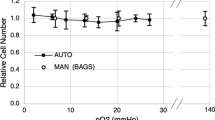
Here, we have studied two parameters critical to process control in mammalian cell culture; dissolved oxygen (dO2) and pH, measured with fluorescent sensors thus allowing the study of the metabolic state of cells in culture without removing or damaging cells during cultivation. Two cell lines, namely, NS0 and CHO were batch-grown in 24-well plates at different serum concentrations with the sensors implemented in the bottom of each well. The data showed a good relationship between the dO2 and pH data obtained from fluorescent probes and the growth and death characteristics of cells. The method has provided a high throughput on-line multi-parametric analysis of mammalian cell cellular activity.




Similar content being viewed by others
References
Arnold SA, Crowley J, Woods N, Harvey LM, McNeil B (2003) In-situ near infrared spectroscopy to monitor key analytes in mammalian cell cultivation. Biotechnol Bioeng 84:13–19. doi:10.1002/bit.10738
Ducommun P, Kadouri A, Von Stockar U, Marison IW (2001) On-line determination of animal cell concentration in two industrial high-density culture processes by dielectric spectroscopy. Biotechnol Bioeng 77:316–323. doi:10.1002/bit.1197
Ge X, Hanson M, Shen H, Kostov Y, Brorson KA, Frey DD et al (2003) Validation of an optical sensor-bases high-throughput bioreactor system for mammalian cell culture. J Biotechnol 122:293–306. doi:10.1016/j.jbiotec.2005.12.009
Hanson MA, Ge X, Kostov Y, Brorson KA, Moreira AR, Rao G (2007) Comparisons of optical pH and dissolved oxygen sensors with traditional electrochemical probes during mammalian cell culture. Biotechnol Bioeng 9:833–841. doi:10.1002/bit.21320
Harding CL, Lloyd DR, McFarlane CM, Al-Rubeai M (2000) Using the microcyte flow cytometer to monitor cell number, viability, and apoptosis in mammalian cell culture. Biotechnol Prog 16:800–802. doi:10.1021/bp0000813
Harms P, Kostov Y, Rao G (2002) Bioprocess monitoring. Curr Opin Biotechnol 13:124–127. doi:10.1016/S0958-1669(02)00295-1
Joeris K, Frerichs J-G, Konstantinov K, Scheper T (2002) In-situ microscopy: online process monitoring of mammalian cell cultures. Cytotechnology 38:129–134. doi:10.1023/A:1021170502775
Konstantinov KB, Pambayun R, Matanguihan R, Yoshida T, Perusich CM, Hu W-S (1992) On-line monitoring of hybridoma cell growth using a laser turbidity sensor. Biotechnol Bioeng 40:1337–1342. doi:10.1002/bit.260401107
Leelavatcharamas V, Emery AN, Al-Rubeai M (1996) Monitoring the proliferative capacity of cultured animal cells by cell cycle analysis. In: Al-Rubeai M, Emery AN (eds) Flow cytometry applications in cell culture. Marcel Dekker Inc., New York, p 1
Miller WM, Blanch HW, Wilke CR (1988) A kinetic analysis of hybridoma growth and metabolism in batch and continuous suspension culture: effect of nutrient concentration, dilution rate, and pH. Biotechnol Bioeng 32:947–965. doi:10.1002/bit.260320803
Mukwena NT, Veraitch F, Al-Rubeai M, Goix P (2003) At-line monitoring of cell cultures: rapid cytometric evaluation of cellular physiology. Guava Technologies. Application note. http://www.gelifesciences.co.jp/technologies/cellular_science/pdf/at_line_monitor.pdf
Perani A, Singh RP, Chauhan R, Al-Rubeai M (1998) Variable functions of bcl-2 in mediating bioreactor stress-induced apoptosis in hybridoma cells. Cytotechnology 28:177–188. doi:10.1023/A:1008002319400
Ozturk SS, Palsson BO (1990) Effects of dissolved oxygen on hybridoma cell growth, metabolism, and antibody production kinetics in continuous culture. Biotechnol Prog 6:437–446. doi:10.1021/bp00006a006
Shah D, Naciri M, Clee P, Al-Rubeai M (2006) NucleoCounter—an efficient technique for the determination of cell number and viability in animal cell culture processes. Cytotechnology 51:39–44. doi:10.1007/s10616-006-9012-9
Schmid G, Blanch HW, Wilke CR (1990) Hybridoma growth, metabolism, and product formation in HEPES-buffered medium: II. Effect of pH. Biotechnol Lett 12:633–638. doi:10.1007/BF01088185
Simpson NH, Milner AE, Al-Rubeai M (1997) Prevention of hybridoma cell death by bcl-2 during sub-optimal culture conditions. Biotechnol Bioeng 54:1–16. doi:10.1002/(SICI)1097-0290(19970405)54:1<1::AID-BIT1>3.0.CO;2-K
Zhao R, Natarajan A, Srienc F (1999) A flow injection flow cytometry system for on-line monitoring of bioreactors. Biotechnol Bioeng 62:609–617. doi:10.1002/(SICI)1097-0290(19990305)62:5<609::AID-BIT13>3.0.CO;2-C
Acknowledgment
This work is funded by a Science Foundation Ireland PI award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naciri, M., Kuystermans, D. & Al-Rubeai, M. Monitoring pH and dissolved oxygen in mammalian cell culture using optical sensors. Cytotechnology 57, 245–250 (2008). https://doi.org/10.1007/s10616-008-9160-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-008-9160-1