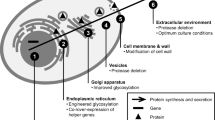
Abstract
Production of recombinant proteins in mammalian cells is a successful technology that delivers protein pharmaceuticals for therapies and for diagnosis of human disorders. Cost effective production of protein biopharmaceuticals requires extensive optimization through cell and fermentation process engineering at the upstream and chemical engineering of purification processes at the downstream side of the production process. The majority of protein pharmaceuticals are secreted proteins. Accumulating evidence suggests that the folding and processing of these proteins in the endoplasmic reticulum (ER) is a general rate- and yield limiting step for their production. We will summarize our knowledge of protein folding in the ER and of signal transduction pathways activated by accumulation of unfolded proteins in the ER, collectively called the unfolded protein response (UPR). On the basis of this knowledge we will evaluate engineering approaches to increase cell specific productivities through engineering of the ER-resident protein folding machinery and of the UPR.








Similar content being viewed by others
Abbreviations
- 4E-BP1:
-
4E-Binding protein 1
- ADP:
-
Adenosine diphosphate
- AKT:
-
AKR/J Mice transforming retroviral oncogene
- AP-1:
-
Activation protein 1
- ASK1:
-
Apoptosis signal-regulating kinase 1
- Asn:
-
L-Asparagine
- ATF4:
-
Activating transcription factor 4
- ATF5:
-
Activating transcription factor 5
- ATF6α:
-
Activating transcription factor 6α
- ATF6β:
-
Activating transcription factor 6β
- ATG5:
-
Autophagy-related 5
- Atg8p:
-
Autophagy-related 8 protein
- ATG12:
-
Autophagy-related 12
- ATG16:
-
Autophagy-related 16
- ATP:
-
Adenosine triphosphate
- BAK:
-
BCL-2 Homologous antagonist/killer
- BAP:
-
BiP-Associated protein
- BAX:
-
BCL-2-Associated X protein
- BBF2:
-
Box B-binding factor 2
- BCL-2:
-
B Cell leukemia/lymphoma 2
- BH1:
-
BCL-2 Homology domain 1
- BH2:
-
BCL-2 Homology domain 2
- BH3:
-
BCL-2 Homology domain 3
- BH4:
-
BCL-2 Homology domain 4
- BIM:
-
BCL-2 Interacting mediator of cell death
- BiP:
-
Heavy chain binding protein
- BMF:
-
BCL-2 Modifying factor
- bZIP:
-
Basic leucine zipper
- cAMP:
-
Cyclic adenosine monophosphate
- CD154:
-
Cluster of differentiation 154
- Cdc48p:
-
Cell division cycle 48 protein
- CDP:
-
Cytidine diphosphate
- CHO:
-
Chinese hamster ovary
- CHOP:
-
CCAAT/Enhancer-binding protein (C/EBP) homologous protein
- cIAP1:
-
Cellular inhibitor of apoptosis 1
- CNC:
-
Cap and collar
- CoA:
-
Coenzyme A
- COPII:
-
Coat Protein II
- CRE:
-
cAMP Response element
- CREB3:
-
CRE-Binding protein 3
- CREB4:
-
CRE-Binding protein 4
- CREB-H:
-
CREB Homolog
- Cue1p:
-
Coupling of ubiquitin conjugation to ER degradation 1 protein
- DD:
-
Death domain
- DED:
-
Death effector domain
- DnaJ:
-
2′-Deoxyribonucleic acid chain elongation J
- DR3:
-
Death receptor 3
- DR6:
-
Death receptor 6
- DTT:
-
1,4-DL-Dithiothreitol
- E 1 :
-
Ubiquitin-activating enzyme
- E 2 :
-
Factor eluted by DTT (ubiquitin-conjugating enzyme)
- E 3 :
-
Eluted with high salt or high pH (ubiquitin ligase)
- ECH:
-
Erythroid cell-derived protein with CNC homology
- EDEM1:
-
ER Degradation enhancer, mannosidase α-like 1
- EDEM2:
-
ER Degradation enhancer, mannosidase α-like 2
- EDEM3:
-
ER Degradation enhancer, mannosidase α-like 3
- eIF2α:
-
Eukaryotic translation initiation factor 2α
- eIF2B:
-
Eukaryotic translation initiation factor 2B
- EIF2AK3:
-
eIF2α Kinase 3
- eIF-4E:
-
Eukaryotic translation initiation factor 4E
- ER:
-
Endoplasmic reticulum
- ERAD:
-
ER Associated protein degradation
- ERdj1:
-
ER DnaJ Protein 1
- ERdj2:
-
ER DnaJ Protein 2
- ERdj3:
-
ER DnaJ Protein 3
- ERdj4:
-
ER DnaJ Protein 4
- ERdj5:
-
ER DnaJ Protein 5
- ERGIC-53:
-
ER Golgi intermediate compartment protein of 53 kDa
- ERK1:
-
Extracellular signal regulated kinase 1
- ERK2:
-
Extracellular signal regulated kinase 2
- ERO1-Lα:
-
Ero1p-like α
- ERO1-Lβ:
-
Ero1p-like β
- Ero1p:
-
ER Oxidation 1 protein
- ERp57:
-
ER Protein of 57 kDa
- ERSE-I:
-
ER Stress response element I
- ERSE-II:
-
ER Stress response element II
- Erv2p:
-
Essential for respiration and viability 2 protein
- FAD:
-
Flavine adenine dinucleotide
- FADD:
-
FAS-Associated protein with a novel DD
- GADD34:
-
Growth arrest and DNA damage gene 34
- GADD45β:
-
Growth arrest and DNA damage gene 45β
- Glc:
-
d-Glucose
- α-Glc I:
-
α-Glucosidase I
- α-Glc II:
-
α-Glucosidase II
- GlcNAC:
-
2-N-Acetyl-d-glucosamine
- GRP78:
-
Glucose-regulated protein of 78 kDa
- GRP94:
-
Glucose-regulated protein of 94 kDa
- GRP170:
-
Glucose-regulated protein of 170 kDa
- GrpE:
-
Growth after phage induction E
- Hac1p:
-
Homologous to ATF/CREB1 1 protein
- HEDJ:
-
Human ER-associated DnaJ
- HERP:
-
Hyperhomocysteinemia-induced ER stress-responsive protein
- hIAP2:
-
Human inhibitor of apoptosis 2
- HMG:
-
3-Hydroxy-3-methylglutarate
- Hrd1p:
-
HMG-CoA reductase degradation 1 protein
- Hrd3p:
-
HMG-CoA Reductase degradation 3 protein
- HSP40:
-
Heat shock protein of 40 kDa
- HSP70:
-
Heat shock protein of 70 kDa
- HSP90:
-
Heat shock protein of 90 kDa
- IAP:
-
Inhibitor of apoptosis
- IgG:
-
Immunoglobulin G
- IκB:
-
Inhibitor of NF-κB
- IKK:
-
IκB Kinase
- IL-2:
-
Interleukin 2
- IL-6:
-
Interleukin 6
- IL-8:
-
Interleukin 8
- IRE1α:
-
Inositol requiring 1α
- IRE1β:
-
Inositol requiring 1β
- IRS-1:
-
Insulin receptor substrate 1
- IRS-2:
-
Insulin receptor substrate 2
- JNK:
-
JUN N-Terminal kinase
- JUN:
-
Ju-nana
- KEAP1:
-
Kelch-like ECH-associated protein 1
- LC3:
-
Microtubule-associated protein 1 light chain 3
- LDLR:
-
Low density lipoprotein receptor
- Lhs1p:
-
Luminal HSP70 1 protein
- m7G:
-
7-Methylguanosine
- Man:
-
D-Mannose
- MAP:
-
Mitogen-activated protein
- MAPKKK:
-
MAP Kinase kinase kinase
- MCP-1:
-
Monocyte chemoattractant protein 1
- MDG1:
-
Microvascular differentiation gene 1
- MEF:
-
Mouse embryonic fibroblast
- MKK7:
-
Mitogen-activated protein kinase kinase 7
- mRNA:
-
Messenger RNA
- mTNF:
-
Membrane-bound TNF
- MTJ1:
-
Murine tumor cell DnaJ-like protein 1
- mTOR:
-
Mammalian TOR
- NADPH:
-
Reduced nicotine adenine dinucleotide phosphate
- NF-κB:
-
Nuclear factor κB
- NOXA:
-
NADPH Oxidase activator
- NRF2:
-
Nuclear factor erythroid 2-related factor 2
- NS0:
-
Non-secreting myeloma cell
- OASIS:
-
Old astrocyte specifically induced substance
- ORP150:
-
150 kDa Oxygen-regulated protein
- p38:
-
Protein of 38 kDa
- p58IPK :
-
58 kDa Inhibitor of PKR
- p65:
-
65 kDa Protein
- p70S6K :
-
70,000 M r 40 S Ribosomal protein S6 kinase
- p97:
-
Protein of 97 kDa
- PDI:
-
Protein disulfide isomerase
- PDK:
-
Phosphoinositide-dependent kinase 2
- PEK:
-
Pancreatic eIF2α kinase
- PERK:
-
PKR-Like ER kinase
- Pi :
-
Inorganic phosphate (HPO4 2−)
- PI:
-
Phosphatidylinositol
- PI3K:
-
PI 3-Kinase
- PKB:
-
Protein kinase B
- PKR:
-
Double-stranded RNA-activated protein kinase
- PP1:
-
Protein phosphatase 1
- PPi :
-
Pyrophosphate (HP2O7 3−)
- PPI:
-
Cis–trans peptidyl prolyl isomerase
- pQC:
-
Preemptive quality control
- PUMA:
-
p53 Upregulated modulator of apoptosis
- Q6:
-
Quiescin 6
- QSCN6:
-
Quiescin 6
- QSCN6L1:
-
Quiescin 6-like 1
- QSOX1:
-
Quiescin Q6 sulfhydryl oxidase 1
- QSOX2:
-
Quiescin Q6 sulfhydryl oxidase 2
- RAS:
-
Rat sarcoma
- RIP:
-
Receptor interacting protein
- RNA:
-
Ribonucleic acid
- RNAi:
-
RNA Interference
- ROS:
-
Reactive oxygen species
- rRNA:
-
Ribosomal RNA
- S1P:
-
Site 1 protease
- S2P:
-
Site 2 protease
- SEC61:
-
Secretory 61
- SEC63:
-
Secretory 63
- Ser:
-
l-Serine
- SERCA:
-
Sarcoplasmic or endoplasmic reticulum Ca2+ ATPase
- SH-2:
-
Src Homology 2 domain
- SHC:
-
SH-2 Domain containing
- SIL1:
-
Suppressor of IRE1/LHS1 synthetic lethality 1
- SODD:
-
Silencer of death domains
- SOXN:
-
Neuroblastoma-derived sulfhydryl oxidase
- Src:
-
Sarcoma formation
- SRE:
-
Sterol response element
- sTNF:
-
Soluble TNF
- TACE:
-
TNF-α Converting enzyme
- Thr:
-
l-Threonine
- TIM:
-
TRAF-Interacting motif
- TNF-α:
-
Tumor necrosis factor α
- TNF-R:
-
TNF-α receptor
- TOR:
-
Target of rapamycin
- TRADD:
-
TNF-R-Associated via DD
- TRAF2:
-
TNF-R Associated factor 2
- TRAIL-R1:
-
TNF-Related apoptosis-inducing ligand receptor 1
- TRAIL-R2:
-
TNF-Related apoptosis-inducing ligand receptor 2
- TRB3:
-
Tribbles homolog 3
- tRNA:
-
Transfer RNA
- TSC1:
-
Tuberous sclerosis complex 1
- TSC2:
-
Tuberous sclerosis complex 2
- Ub:
-
Ubiquitin
- Ubc7p:
-
Ubiquitin-conjugating enzyme 7 protein
- UDP:
-
Uridine diphosphate
- UGGT:
-
UDP-Glucose:glycoprotein glucosyltransferase
- UPR:
-
Unfolded protein response
- VCP:
-
Valosin-containing protein
- WT:
-
Wild type
- XBP-1:
-
X-Box-binding protein 1
- Yos9p:
-
Yeast osteosarcoma 9 homolog protein
References
Ailor EN, Reff ME (2005) Method to increase protein production in culture. US Patent 2005/106,222, 9 May 2005
Al-Sheikh H, Watson AJ, Lacey GA, Punt PJ, MacKenzie DA, Jeenes DJ et al (2004) Endoplasmic reticulum stress leads to the selective transcriptional downregulation of the glucoamylase gene in Aspergillus niger. Mol Microbiol 53:1731–1742. doi :10.1111/j.1365-2958.2004.04236.x
Alberti S, Esser C, Höhfeld J (2003) BAG-1—a nucleotide exchange factor of Hsc70 with multiple cellular functions. Cell Stress Chaperones 8:225–231. doi :10.1379/1466-1268(2003)008<0225:BNEFOH>2.0.CO;2
Alete DE, Racher AJ, Birch JR, Stansfield SH, James DC, Smales CM (2005) Proteomic analysis of enriched microsomal fractions from GS-NS0 murine myeloma cells with varying secreted recombinant monoclonal antibody productivities. Proteomics 5:4689–4704. doi:10.1002/pmic.200500019
Baeuerle PA, Henkel T (1994) Function and activation of NF-κB in the immune system. Annu Rev Immunol 12:141–179. doi:10.1146/annurev.iy.12.040194.001041
Barnes LM, Bentley CM, Dickson AJ (2004) Molecular definition of predictive indicators of stable protein expression in recombinant NS0 myeloma cells. Biotechnol Bioeng 85:115–121. doi:10.1002/bit.10893
Bernales S, Papa FR, Walter P (2006) Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol 22:487–508. doi:10.1146/annurev.cellbio.21.122303.120200
Biswas C, Ostrovsky O, Makarewich CA, Wanderling S, Gidalevitz T, Argon Y (2007) The peptide binding activity of GRP94 is regulated by calcium. Biochem J 405:233–241. doi:10.1042/BJ20061867
Bonifacino JS, Glick BS (2004) The mechanisms of vesicle budding and fusion. Cell 116:153–166. doi:10.1016/S0092-8674(03)01079-1
Chung JY, Lim SW, Hong YJ, Hwang SO, Lee GM (2004) Effect of doxycycline-regulated calnexin and calreticulin expression on specific thrombopoietin productivity of recombinant Chinese hamster ovary cells. Biotechnol Bioeng 85:539–546. doi:10.1002/bit.10919
Cudna RE, Dickson AJ (2006) Engineering responsiveness to cell culture stresses: Growth arrest and DNA damage gene 153 (GADD153) and the unfolded protein response (UPR) in NS0 myeloma cells. Biotechnol Bioeng 94:514–521. doi:10.1002/bit.20861
Cullinan SB, Diehl JA (2006) Coordination of ER and oxidative stress signaling: The PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol 38:317–332. doi:10.1016/j.biocel.2005.09.018
de StGroth SF, Scheidegger D (1980) Production of monoclonal antibodies: strategy and tactics. J Immunol Methods 35:1–21. doi:10.1016/0022-1759(80)90146-5
Dejgaard S, Nicolay J, Taheri M, Thomas DY, Bergeron JJ (2004) The ER glycoprotein quality control system. Curr Issues Mol Biol 6:29–42
Dempsey PW, Doyle SE, He JQ, Cheng G (2003) The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev 14:193–209. doi:10.1016/S1359-6101(03)00021-2
Ding W-X, Ni H-M, Gao W, Hou Y-F, Melan MA, Chen X et al (2007) Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem 282:4702–4710. doi:10.1074/jbc.M609267200
Dinnis DM, James DC (2005) Engineering mammalian cell factories for improved recombinant monoclonal antibody production: lessons from nature? Biotechnol Bioeng 91:180–189. doi:10.1002/bit.20499
Dollins DE, Warren JJ, Immormino RM, Gewirth DT (2007) Structures of GRP94-nucleotide complexes reveal mechanistic differences between the hsp90 chaperones. Mol Cell 28:41–56. doi:10.1016/j.molcel.2007.08.024
Doolan P, Melville M, Gammell P, Sinacore M, Meleady P, McCarthy K et al (2008) Transcriptional profiling of gene expression changes in a PACE-transfected CHO DUKX cell line secreting high levels of rhBMP–2. Mol Biotechnol 39:187–199. doi:10.1007/s12033-008-9039-6
Dorner AJ, Kaufman RJ (1994) The levels of endoplasmic reticulum proteins and ATP affect folding and secretion of selective proteins. Biologicals 22:103–112. doi:10.1006/biol.1994.1016
Dorner AJ, Bole DG, Kaufman RJ (1987) The relationship of N-linked glycosylation and heavy chain-binding protein association with the secretion of glycoproteins. J Cell Biol 105:2665–2674. doi:10.1083/jcb.105.6.2665
Dorner AJ, Krane MG, Kaufman RJ (1988) Reduction of endogenous GRP78 levels improves secretion of a heterologous protein in CHO cells. Mol Cell Biol 8:4063–4070
Dorner AJ, Wasley LC, Kaufman RJ (1989) Increased synthesis of secreted proteins induces expression of glucose-regulated proteins in butyrate-treated Chinese hamster ovary cells. J Biol Chem 264:20602–20607
Dorner AJ, Wasley LC, Kaufman RJ (1992) Overexpression of GRP78 mitigates stress induction of glucose regulated proteins and blocks secretion of selective proteins in Chinese hamster ovary cells. EMBO J 11:1563–1571
Downham MR, Farrell WE, Jenkins HA (1996) Endoplasmic reticulum protein expression in recombinant NS0 myelomas grown in batch culture. Biotechnol Bioeng 51:691–696. doi :10.1002/(SICI)1097-0290(19960920)51:6<691::AID-BIT7>3.0.CO;2-C
Draznin B (2006) Molecular mechanisms of insulin resistance: serine phosphorylation of insulin receptor substrate-1 and increased expression of p85alpha: the two sides of a coin. Diabetes 55:2392–2397. doi:10.2337/db06-0391
Du K, Herzig S, Kulkarni RN, Montminy M (2003) TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 300:1574–1577. doi:10.1126/science.1079817
Ellgaard L, Ruddock LW (2005) The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO Rep 6:28–32. doi:10.1038/sj.embor.7400311
Fann CH, Guarna MM, Kilburn DG, Piret JM (1999) Relationship between recombinant activated protein C secretion rates and mRNA levels in baby hamster kidney cells. Biotechnol Bioeng 63:464–472. doi : 10.1002/(SICI)1097-0290(19990520)63:4<464::AID-BIT10>3.0.CO;2-H
Fisher EA, Zhou M, Mitchell DM, Wu X, Omura S, Wang H et al (1997) The degradation of apolipoprotein B100 is mediated by the ubiquitin-proteasome pathway and involves heat shock protein 70. J Biol Chem 272:20427–20434. doi:10.1074/jbc.272.33.20427
Frey S, Leskovar A, Reinstein J, Buchner J (2007) The ATPase cycle of the endoplasmic chaperone Grp94. J Biol Chem 282:35612–35620. doi:10.1074/jbc.M704647200
Friedlander R, Jarosch E, Urban J, Volkwein C, Sommer T (2000) A regulatory link between ER-associated protein degradation and the unfolded-protein response. Nat Cell Biol 2:379–384. doi:10.1038/35017001
Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A et al (2006) The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol 26:2490–2496. doi:10.1161/01.ATV.0000242903.41158.a1
Gennaro DE, Hoffstein ST, Marks G, Ramos L, Oka MS, Reff ME et al (1991) Quantitative immunocytochemical staining for recombinant tissue-type plasminogen activator in transfected Chinese hamster ovary cells. Proc Soc Exp Biol Med 198:591–598
Gething M-J, McCammon K, Sambrook J (1986) Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell 46:939–950. doi:10.1016/0092-8674(86)90076-0
Gusarova V, Caplan AJ, Brodsky JL, Fisher EA (2001) Apoprotein B degradation is promoted by the molecular chaperones hsp90 and hsp70. J Biol Chem 276:24891–24900. doi:10.1074/jbc.M100633200
Häcker G, Weber A (2007) BH3-only proteins trigger cytochrome c release, but how? Arch Biochem Biophys 462:150–155. doi:10.1016/j.abb.2006.12.022
Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M et al (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11:619–633. doi:10.1016/S1097-2765(03)00105-9
Hasemann CA, Capra JD (1990) High-level production of a functional immunoglobulin heterodimer in a baculovirus expression system. Proc Natl Acad Sci USA 87:3942–3946. doi:10.1073/pnas.87.10.3942
Hayano T, Hirose M, Kikuchi M (1995) Protein disulfide isomerase mutant lacking its isomerase activity accelerates protein folding in the cell. FEBS Lett 377:505–511. doi:10.1016/0014-5793(95)01410-1
Haynes CM, Titus EA, Cooper AA (2004) Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell 15:767–776. doi:10.1016/j.molcel.2004.08.025
Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B et al (2006) Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1α. Science 312:572–576. doi:10.1126/science.1123480
Hippenmeyer P, Highkin M (1993) High level, stable production of recombinant proteins in mammalian cell culture using the herpesvirus VP16 transactivator. Biotechnology (N Y) 11:1037–1041. doi:10.1038/nbt0993-1037
Hollien J, Weissman JS (2006) Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313:104–107. doi:10.1126/science.1129631
Hsu T-A, Betenbaugh MJ (1997) Coexpression of molecular chaperone BiP improves immunoglobulin solubility and IgG secretion from Trichoplusia ni insect cells. Biotechnol Prog 13:96–104. doi:10.1021/bp960088d
Hsu T-A, Watson S, Eiden JJ, Betenbaugh MJ (1996) Rescue of immunoglobulins from insolubility is facilitated by PDI in the baculovirus expression system. Protein Expr Purif 7:281–288. doi:10.1006/prep. 1996.0040
Hsu TA, Eiden JJ, Bourgarel P, Meo T, Betenbaugh MJ (1994) Effects of co-expressing chaperone BiP on functional antibody production in the baculovirus system. Protein Expr Purif 5:595–603. doi:10.1006/prep. 1994.1082
Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH (2006) Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-κB activation and down-regulation of TRAF2 expression. Mol Cell Biol 26:3071–3084. doi:10.1128/MCB.26.8.3071-3084.2006
Hurtley SM, Bole DG, Hoover-Litty H, Helenius A, Copeland CS (1989) Interactions of misfolded influenza virus hemagglutinin with binding protein (BiP). J Cell Biol 108:2117–2126. doi:10.1083/jcb.108.6.2117
Imagawa Y, Hosoda A, Sasaka S-i, Tsuru A, Kohno K (2008) RNase domains determine the functional difference between IRE1α and IRE1β. FEBS Lett 582:656–660. doi:10.1016/j.febslet.2008.01.038
Iwawaki T, Hosoda A, Okuda T, Kamigori Y, Nomura-Furuwatari C, Kimata Y et al (2001) Translational control by the ER transmembrane kinase/ribonuclease IRE1 under ER stress. Nat Cell Biol 3:158–164. doi:10.1038/35055065
Jarvis DL, Summers MD (1989) Glycosylation and secretion of human tissue plasminogen activator in recombinant baculovirus-infected insect cells. Mol Cell Biol 9:214–223
Jeon HK, Chang KH, Kim KI, Chung IS (2003) Functional expression of recombinant tumstatin in stably transformed Drosophila melanogaster S2 cells. Biotechnol Lett 25:185–189. doi:10.1023/A:1022330429508
Jones J, Nivitchanyong T, Giblin C, Ciccarone V, Judd D, Gorfien S et al (2005) Optimization of tetracycline-responsive recombinant protein production and effect on cell growth and ER stress in mammalian cells. Biotechnol Bioeng 91:722–732. doi:10.1002/bit.20566
Kang S-W, Rane NS, Kim SJ, Garrison JL, Taunton J, Hegde RS (2006) Substrate-specific translocational attenuation during ER stress defines a pre-emptive quality control pathway. Cell 127:999–1013. doi:10.1016/j.cell.2006.10.032
Karst AM, Li G (2007) BH3-only proteins in tumorigenesis and malignant melanoma. Cell Mol Life Sci 64:318–330. doi:10.1007/s00018-006-6364-4
Kato T, Murata T, Usui T, Park EY (2005) Improvement of the production of GFPuv-β1, 3-N-acetylglucosaminyltransferase 2 fusion protein using a molecular chaperone-assisted insect-cell-based expression system. Biotechnol Bioeng 89:424–433. doi:10.1002/bit.20362
Kaufman RJ (2004) Regulation of mRNA translation by protein folding in the endoplasmic reticulum. Trends Biochem Sci 29:152–158. doi:10.1016/j.tibs.2004.01.004
Kaufman RJ, Wasley LC, Dorner AJ (1988) Synthesis, processing, and secretion of recombinant human factor VIII expressed in mammalian cells. J Biol Chem 263:6352–6362
Kimata Y, Ishiwata-Kimata Y, Yamada S, Kohno K (2006) Yeast unfolded protein response pathway regulates expression of genes for anti-oxidative stress and for cell surface proteins. Genes Cells 11:59–69. doi:10.1111/j.1365-2443.2005.00921.x
Kitchin K, Flickinger MC (1995) Alteration of hybridoma viability and antibody secretion in transfectomas with inducible overexpression of protein disulfide isomerase. Biotechnol Prog 11:565–574. doi:10.1021/bp00035a011
Klionsky DJ (2005) The molecular machinery of autophagy: unanswered questions. J Cell Sci 118:7–18. doi:10.1242/jcs.01620
Kohno K, Normington K, Sambrook J, Gething MJ, Mori K (1993) The promoter region of the yeast KAR2 (BiP) gene contains a regulatory domain that responds to the presence of unfolded proteins in the endoplasmic reticulum. Mol Cell Biol 13:877–890
Kondo S, Saito A, Hino SI, Murakami T, Ogata M, Kanemoto S et al (2007) BBF2H7, a novel transmembrane bZIP transcription factor, is a new type of ER stress transducer. Mol Cell Biol 27:1716–1729. doi:10.1128/MCB.01552-06
Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H et al (2007) ER stress (PERK/eIF2α phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ 14:230–239. doi:10.1038/sj.cdd.4401984
Ku SCY, Ng DTW, Yap MGS, Chao S-H (2008) Effects of overexpression of X-box binding protein 1 on recombinant protein production in Chinese hamster ovary and NS0 myeloma cells. Biotechnol Bioeng 99:155–164. doi:10.1002/bit.21562
Lambert N, Merten OW (1997) Effect of serum-free and serum-containing medium on cellular levels of ER-based proteins in various mouse hybridoma cell lines. Biotechnol Bioeng 54:165–180. doi : 10.1002/(SICI)1097-0290(19970420)54:2<165::AID-BIT8>3.0.CO;2-J
Lederkremer GZ, Glickman MH (2005) A window of opportunity: timing protein degradation by trimming of sugars and ubiquitins. Trends Biochem Sci 30:297–303. doi:10.1016/j.tibs.2005.04.010
Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23:7448–7459. doi:10.1128/MCB.23.21.7448-7459.2003
Lee JM, Chang KH, Park JH, Lee YH, Chung IS (2001) Production of recombinant endostatin from stably transformed Trichoplusia ni BTI Tn 5B1-4 cells. Biotechnol Lett 23:1931–1936. doi:10.1023/A:1013726113708
Li Y, Ge M, Ciani L, Kuriakose G, Westover EJ, Dura M et al (2004) Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids: implications for depletion of endoplasmic reticulum calcium stores and apoptosis in cholesterol-loaded macrophages. J Biol Chem 279:37030–37039. doi:10.1074/jbc.M405195200
Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B et al (2007) IRE1 signaling affects cell fate during the unfolded protein response. Science 318:944–949. doi:10.1126/science.1146361
Machamer CE, Doms RW, Bole DG, Helenius A, Rose JK (1990) Heavy chain binding protein recognizes incompletely disulfide-bonded forms of vesicular stomatitis virus G protein. J Biol Chem 265:6879–6883
Marciniak SJ, Ron D (2006) Endoplasmic reticulum stress signaling in disease. Physiol Rev 86:1133–1149. doi:10.1152/physrev.00015.2006
Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R et al (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18:3066–3077. doi:10.1101/gad.1250704
Martínez IM, Chrispeels MJ (2003) Genomic analysis of the unfolded protein response in Arabidopsis shows its connection to important cellular processes. Plant Cell 15:561–576. doi:10.1105/tpc.007609
Mauro C, Crescenzi E, De Mattia R, Pacifico F, Mellone S, Salzano S et al (2006) Central role of the scaffold protein tumor necrosis factor receptor-associated factor 2 in regulating endoplasmic reticulum stress-induced apoptosis. J Biol Chem 281:2631–2638. doi:10.1074/jbc.M502181200
McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21:1249–1259. doi:10.1128/MCB.21.4.1249-1259.2001
Meusser B, Hirsch C, Jarosch E, Sommer T (2005) ERAD: the long road to destruction. Nat Cell Biol 7:766–772. doi:10.1038/ncb0805-766
Miura T, Katakura Y, Seto P, Zhang YP, Teruya K, Nishimura E et al (2001) Availability of oncogene activated production system for mass production of light chain of human antibody in CHO cells. Cytotechnology 35:9–16. doi:10.1023/A:1008179919857
Mohan C, Park SH, Chung JY, Lee GM (2007) Effect of doxycycline-regulated protein disulfide isomerase expression on the specific productivity of recombinant CHO cells: thrombopoietin and antibody. Biotechnol Bioeng 98:611–615. doi:10.1002/bit.21453
Morano KA (2007) New tricks for an old dog: the evolving world of Hsp70. Ann N Y Acad Sci 1113:1–14. doi:10.1196/annals.1391.018
Moremen KW, Molinari M (2006) N-linked glycan recognition and processing: the molecular basis of endoplasmic reticulum quality control. Curr Opin Struct Biol 16:592–599. doi:10.1016/j.sbi.2006.08.005
Morris JA, Dorner AJ, Edwards CA, Hendershot LM, Kaufman RJ (1997) Immunoglobulin binding protein (BiP) function is required to protect cells from endoplasmic reticulum stress but is not required for the secretion of selective proteins. J Biol Chem 272:4327–4334. doi:10.1074/jbc.272.7.4327
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA et al (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 403:98–103. doi:10.1038/47513
Nichols WC, Seligsohn U, Zivelin A, Terry VH, Hertel CE, Wheatley MA et al (1998) Mutations in the ER-Golgi intermediate compartment protein ERGIC-53 cause combined deficiency of coagulation factors V and VIII. Cell 93:61–70. doi:10.1016/S0092-8674(00)81146-0
Nishikawa S, Hirata A, Nakano A (1994) Inhibition of endoplasmic reticulum (ER)-to-Golgi transport induces relocalization of binding protein (BiP) within the ER to form the BiP bodies. Mol Biol Cell 5:1129–1143
Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K et al (2002) ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev 16:1345–1355. doi:10.1101/gad.992302
Ogata M, Hino SI, Saito A, Morikawa K, Kondo S, Kanemoto S et al (2006) Autophagy is activated for cell survival after ER stress. Mol Cell Biol 26:9220–9231. doi:10.1128/MCB.01453-06
Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H (2005) TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J 24:1243–1255. doi:10.1038/sj.emboj.7600596
Ohya T, Hayashi T, Kiyama E, Nishii H, Miki H, Kobayashi K et al (2007) Improved production of recombinant human antithrombin III in Chinese hamster ovary cells by ATF4 overexpression. Biotechnol Bioeng 100:317–324. doi:10.1002/bit.21758
Özcan U, Cao Q, Yilmaz E, Lee A-H, Iwakoshi NN, Ozdelen E et al (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306:457–461. doi:10.1126/science.1103160
Ozcan U, Ozcan L, Yilmaz E, Düvel K, Sahin M, Manning BD et al (2008) Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell 29:541–551. doi:10.1016/j.molcel.2007.12.023
Pakula TM, Laxell M, Huuskonen A, Uusitalo J, Saloheimo M, Penttilä M (2003) The effects of drugs inhibiting protein secretion in the filamentous fungus Trichoderma reesei. Evidence for down-regulation of genes that encode secreted proteins in the stressed cells. J Biol Chem 278:45011–45020. doi:10.1074/jbc.M302372200
Papa S, Zazzeroni F, Bubici C, Jayawardena S, Alvarez K, Matsuda S et al (2004) Gadd45β mediates the NF-κB suppression of JNK signalling by targeting MKK7/JNKK2. Nat Cell Biol 6:146–153. doi:10.1038/ncb1093
Parekh R, Forrester K, Wittrup D (1995) Multicopy overexpression of bovine pancreatic trypsin inhibitor saturates the protein folding and secretory capacity of Saccharomyces cerevisiae. Protein Expr Purif 6:537–545. doi:10.1006/prep. 1995.1071
Park JH, Lee JM, Chung IS (1999) Production of recombinant endostatin from stably transformed Drosophila melanogaster S2 cells. Biotechnol Lett 21:729–733. doi:10.1023/A:1005510821928
Pendse GJ, Karkare S, Bailey JE (1992) Effect of cloned gene dosage on cell growth and hepatitis B surface antigen synthesis and secretion in recombinant CHO cells. Biotechnol Bioeng 40:119–129. doi:10.1002/bit.260400117
Perlmutter DH (2006) The role of autophagy in alpha–1-antitrypsin deficiency: a specific cellular response in genetic diseases associated with aggregation-prone proteins. Autophagy 2:258–263
Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K et al (2004) Ferritin heavy chain upregulation by NF-κB inhibits TNFα-induced apoptosis by suppressing reactive oxygen species. Cell 119:529–542. doi:10.1016/j.cell.2004.10.017
Randall TD, Parkhouse RM, Corley RB (1992) J chain synthesis and secretion of hexameric IgM is differentially regulated by lipopolysaccharide and interleukin 5. Proc Natl Acad Sci USA 89:962–966. doi:10.1073/pnas.89.3.962
Randow F, Seed B (2001) Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat Cell Biol 3:891–896. doi:10.1038/ncb1001-891
Robinson DK, Memmert KW (1991) Kinetics of recombinant immunoglobulin production by mammalian-cells in continuous culture. Biotechnol Bioeng 38:972–976. doi:10.1002/bit.260380903
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529. doi:10.1038/nrm2199
Rosser MF, Trotta BM, Marshall MR, Berwin B, Nicchitta CV (2004) Adenosine nucleotides and the regulation of GRP94-client protein interactions. Biochemistry 43:8835–8845. doi:10.1021/bi049539q
Roux PP, Blenis J (2004) ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68:320–344. doi:10.1128/MMBR.68.2.320-344.2004
Sakaki K, Wu J, Kaufman RJ (2008) Protein kinase Cθ is required for autophagy in response to stress in the endoplasmic reticulum. J Biol Chem 283:15370–15380. doi:10.1074/jbc.M710209200
Saltiel AR, Kahn CR (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799–806. doi:10.1038/414799a
Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P et al (2001) Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell 7:1165–1176. doi:10.1016/S1097-2765(01)00265-9
Schröder M (2006) The unfolded protein response. Mol Biotechnol 34:279–290. doi:10.1385/MB:34:2:279
Schröder M (2008a) Endoplasmic reticulum stress responses. Cell Mol Life Sci 65:862–894. doi:10.1007/s00018-007-7383-5
Schröder M (2008b) Engineering eukaryotic protein factories. Biotechnol Lett 30:187–196. doi:10.1007/s10529-007-9524-1
Schröder M, Friedl P (1997) Overexpression of recombinant human antithrombin III in Chinese hamster ovary cells results in malformation and decreased secretion of the recombinant protein. Biotechnol Bioeng 53:547–559. doi :10.1002/(SICI)1097-0290(19970320)53:6<547::AID-BIT2>3.0.CO;2-M
Schröder M, Kaufman RJ (2005a) ER stress and the unfolded protein response. Mutat Res 569:29–63. doi:10.1016/j.mrfmmm.2004.06.056
Schröder M, Kaufman RJ (2005b) The mammalian unfolded protein response. Annu Rev Biochem 74:739–789. doi:10.1146/annurev.biochem.73.011303.074134
Schröder M, Kaufman RJ (2006) Divergent roles of IRE1α and PERK in the unfolded protein response. Curr Mol Med 6:5–36. doi:10.2174/156652406775574569
Schröder M, Körner C, Friedl P (1999) Quantitative analysis of transcription and translation in gene amplified Chinese hamster ovary cells on the basis of a kinetic model. Cytotechnology 29:93–102. doi:10.1023/A:1008077603328
Schröder M, Schäfer R, Friedl P (2002) Induction of protein aggregation in an early secretory compartment by elevation of expression level. Biotechnol Bioeng 78:131–140. doi:10.1002/bit.10206
Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee A-H, Qian SB, Zhao H et al (2004) XBP1, downstream of Blimp–1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21:81–93. doi:10.1016/j.immuni.2004.06.010
Shah OJ, Wang Z, Hunter T (2004) Inappropriate activation of the TSC/Rheb/mTOR/S6 K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol 14:1650–1656. doi:10.1016/j.cub.2004.08.026
Shulman GI (1999) Cellular mechanisms of insulin resistance in humans. Am J Cardiol 84:3J–10J. doi:10.1016/S0002-9149(99)00350-1
Smales CM, Dinnis DM, Stansfield SH, Alete D, Sage EA, Birch JR et al (2004) Comparative proteomic analysis of GS-NS0 murine myeloma cell lines with varying recombinant monoclonal antibody production rate. Biotechnol Bioeng 88:474–488. doi:10.1002/bit.20272
Smith JD, Tang BC, Robinson AS (2004) Protein disulfide isomerase, but not binding protein, overexpression enhances secretion of a non-disulfide-bonded protein in yeast. Biotechnol Bioeng 85:340–350. doi:10.1002/bit.10853
Sriburi R, Jackowski S, Mori K, Brewer JW (2004) XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol 167:35–41. doi:10.1083/jcb.200406136
Steel GJ, Fullerton DM, Tyson JR, Stirling CJ (2004) Coordinated activation of Hsp70 chaperones. Science 303:98–101. doi:10.1126/science.1092287
Strudwick N, Schröder M (2007) The unfolded protein response. In: Al-Rubeai M, Fussenegger M (eds) Systems biology. Cell engineering, vol 5. Springer Verlag, Dordrecht, pp 69–157
Suzuki CK, Bonifacino JS, Lin AY, Davis MM, Klausner RD (1991) Regulating the retention of T-cell receptor α chain variants within the endoplasmic reticulum: Ca2+-dependent association with BiP. J Cell Biol 114:189–205. doi:10.1083/jcb.114.2.189
Tanida I, Ueno T, Kominami E (2004) LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol 36:2503–2518. doi:10.1016/j.biocel.2004.05.009
Teruya K, Daimon Y, Dong XY, Katakura Y, Miura T, Ichikawa A et al (2005) An approach to further enhance the cellular productivity of exogenous protein hyper-producing Chinese hamster ovary (CHO) cells. Cytotechnology 47:29–36. doi:10.1007/s10616-005-3765-4
Thorpe C, Coppock DL (2007) Generating disulfides in multicellular organisms: emerging roles for a new flavoprotein family. J Biol Chem 282:13929–13933. doi:10.1074/jbc.R600037200
Thuerauf DJ, Marcinko M, Belmont PJ, Glembotski CC (2007) Effects of the isoform-specific characteristics of ATF6α and ATF6β on endoplasmic reticulum stress response gene expression and cell viability. J Biol Chem 282:22865–22878. doi:10.1074/jbc.M701213200
Tigges M, Fussenegger M (2006) Xbp1-based engineering of secretory capacity enhances the productivity of Chinese hamster ovary cells. Metab Eng 8:264–272. doi:10.1016/j.ymben.2006.01.006
Tu BP, Weissman JS (2002) The FAD- and O2-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol Cell 10:983–994. doi:10.1016/S1097-2765(02)00696-2
Tu BP, Weissman JS (2004) Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol 164:341–346. doi:10.1083/jcb.200311055
Underhill MF, Coley C, Birch JR, Findlay A, Kallmeier R, Proud CG et al (2003) Engineering mRNA translation initiation to enhance transient gene expression in chinese hamster ovary cells. Biotechnol Prog 19:121–129. doi:10.1021/bp025560b
van Anken E, Romijn EP, Maggioni C, Mezghrani A, Sitia R, Braakman I et al (2003) Sequential waves of functionally related proteins are expressed when B cells prepare for antibody secretion. Immunity 18:243–253. doi:10.1016/S1074-7613(03)00024-4
van der Heide M, Hollenberg CP, van der Klei IJ, Veenhuis M (2002) Overproduction of BiP negatively affects the secretion of Aspergillus niger glucose oxidase by the yeast Hansenula polymorpha. Appl Microbiol Biotechnol 58:487–494. doi:10.1007/s00253-001-0907-2
Veal EA, Day AM, Morgan BA (2007) Hydrogen peroxide sensing and signaling. Mol Cell 26:1–14. doi:10.1016/j.molcel.2007.03.016
Wajant H, Scheurich P (2001) Tumor necrosis factor receptor-associated factor (TRAF) 2 and its role in TNF signaling. Int J Biochem Cell Biol 33:19–32. doi:10.1016/S1357-2725(00)00064-9
Warnakulasuriyarachchi D, Cerquozzi S, Cheung HH, Holcik M (2004) Translational induction of the inhibitor of apoptosis protein HIAP2 during endoplasmic reticulum stress attenuates cell death and is mediated via an inducible internal ribosome entry site element. J Biol Chem 279:17148–17157. doi:10.1074/jbc.M308737200
Watowich SS, Morimoto RI, Lamb RA (1991) Flux of the paramyxovirus hemagglutinin-neuraminidase glycoprotein through the endoplasmic reticulum activates transcription of the GRP78-BiP gene. J Virol 65:3590–3597
Welker E, Wedemeyer WJ, Narayan M, Scheraga HA (2001) Coupling of conformational folding and disulfide-bond reactions in oxidative folding of proteins. Biochemistry 40:9059–9064. doi:10.1021/bi010409g
Werner RG (2004) Economic aspects of commercial manufacture of biopharmaceuticals. J Biotechnol 113:171–182. doi:10.1016/j.jbiotec.2004.04.036
White MF (2002) IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab 283:E413–E422. doi:10.1152/ajpendo.00514.2001
Whiteley EM, Hsu T-A, Betenbaugh MJ (1997) Modeling assembly, aggregation, and chaperoning of immunoglobulin G production in insect cells. Biotechnol Bioeng 56:106–116. doi :10.1002/(SICI)1097-0290(19971005)56:1<106::AID-BIT12>3.0.CO;2-I
Wiest DL, Burkhardt JK, Hester S, Hortsch M, Meyer DI, Argon Y (1990) Membrane biogenesis during B cell differentiation: most endoplasmic reticulum proteins are expressed coordinately. J Cell Biol 110:1501–1511. doi:10.1083/jcb.110.5.1501
Williams DB (2006) Beyond lectins: the calnexin/calreticulin chaperone system of the endoplasmic reticulum. J Cell Sci 119:615–623. doi:10.1242/jcs.02856
Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J et al (2007) ATF6α optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell 13:351–364. doi:10.1016/j.devcel.2007.07.005
Wurm FM (2004) Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol 22:1393–1398. doi:10.1038/nbt1026
Xu C, Bailly-Maitre B, Reed JC (2005) Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115:2656–2664. doi:10.1172/JCI26373
Xu X, Azakami H, Kato A (2004) P-domain and lectin site are involved in the chaperone function of Saccharomyces cerevisiae calnexin homologue. FEBS Lett 570:155–160. doi:10.1016/j.febslet.2004.06.039
Yamaguchi S, Ishihara H, Yamada T, Tamura A, Usui M, Tominaga R et al (2008) ATF4-mediated induction of 4E-BP1 contributes to pancreatic β cell survival under endoplasmic reticulum stress. Cell Metab 7:269–276. doi:10.1016/j.cmet.2008.01.008
Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H et al (2007) Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Dev Cell 13:365–376. doi:10.1016/j.devcel.2007.07.018
Yokouchi M, Hiramatsu N, Hayakawa K, Okamura M, Du S, Kasai A et al (2008) Involvement of selective reactive oxygen species upstream of proapoptotic branches of unfolded protein response. J Biol Chem 283:4252–4260. doi:10.1074/jbc.M705951200
Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T et al (2001) Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem 276:13935–13940. doi:10.1074/jbc.M010677200
Yoshida H, Matsui T, Hosokawa N, Kaufman RJ, Nagata K, Mori K (2003) A time-dependent phase shift in the mammalian unfolded protein response. Dev Cell 4:265–271. doi:10.1016/S1534-5807(03)00022-4
Yoshimura FK, Luo X, Zhao X, Gerard HC, Hudson AP (2008) Up-regulation of a cellular protein at the translational level by a retrovirus. Proc Natl Acad Sci USA 105:5543–5548. doi:10.1073/pnas.0710526105
Zang M, Trautmann H, Gandor C, Messi F, Asselbergs F, Leist C et al (1995) Production of recombinant proteins in Chinese hamster ovary cells using a protein-free cell culture medium. Biotechnology (N Y) 13:389–392. doi:10.1038/nbt0495-389
Zeng L, Lu M, Mori K, Luo S, Lee AS, Zhu Y et al (2004) ATF6 modulates SREBP2-mediated lipogenesis. EMBO J 23:950–958. doi:10.1038/sj.emboj.7600106
Zhang K, Wong HN, Song B, Miller CN, Scheuner D, Kaufman RJ (2005) The unfolded protein response sensor IRE1α is required at 2 distinct steps in B cell lymphopoiesis. J Clin Invest 115:268–281
Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT et al (2006) Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 124:587–599. doi:10.1016/j.cell.2005.11.040
Zhou D, Pallam LR, Jiang L, Narasimhan J, Staschke KA, Wek RC (2008) Phosphorylation of eIF2 directs ATF5 translational control in response to diverse stress conditions. J Biol Chem 283:7064–7073. doi:10.1074/jbc.M708530200
Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H et al (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12:982–995. doi:10.1101/gad.12.7.982
Acknowledgements
We apologize to all whose work could not be cited because of space limitations. This work was supported by funding from the Biotechnology and Biological Sciences Research Council (C513418/1, D01588X/1, E006035/1), the European Commission (HEALTH-F7-2007-201608), and the Wellcome Trust (079821) to M.S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, S.U., Schröder, M. Engineering of chaperone systems and of the unfolded protein response. Cytotechnology 57, 207–231 (2008). https://doi.org/10.1007/s10616-008-9157-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-008-9157-9