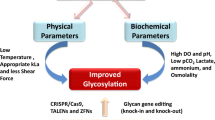
Abstract
Many biopharmaceuticals are now produced as secreted glycoproteins from mammalian cell culture. The glycosylation profile of these proteins is essential to ensure structural stability and biological and clinical activity. However, the ability to control the glycosylation is limited by our understanding of the parameters that affect the heterogeneity of added glycan structures. It is clear that the glycosylation process is affected by a number of factors including the 3-dimensional structure of the protein, the enzyme repertoire of the host cell, the transit time in the Golgi and the availability of intracellular sugar-nucleotide donors. From a process development perspective there are many culture parameters that can be controlled to enable a consistent glycosylation profile to emerge from each batch culture. A further, but more difficult goal is to control the culture conditions to enable the enrichment of specific glycoforms identified with desirable biological activities. The purpose of this paper is to discuss the cellular metabolism associated with protein glycosylation and review the attempts to manipulate, control or engineer this metabolism to allow the expression of human glycosylation profiles in producer lines such as genetically engineered Chinese hamster ovary (CHO) cells.
Similar content being viewed by others
Abbreviations
- ADCC:
-
antibody-mediated cytotoxicity
- BHK:
-
baby hamster kidney (cells)
- C2GnT:
-
core 2 GlcNAC transferase (UDP-GlcNAc: Galβ1,3GalNAc-R β1,6-N-acetyl glucosaminyl transferase)
- CHO:
-
Chinese hamster ovary (cells)
- CMP:
-
cytidine monophosphate
- DO:
-
dissolved oxygen
- EPO:
-
erythropoietin
- ER:
-
endoplasmic reticulum
- FT:
-
fucosyl transferase
- G0:
-
agalactosylated glycans
- G1:
-
monogalactosylated glycans
- G2:
-
digalactosylated glycans
- GalNAc:
-
N-acetyl galactosamine
- GDM:
-
GDP mannose 4,6 dehydratase
- GDP:
-
guanosine diphosphate
- GFAT:
-
glutamine: fructose 6-phosphate amidotransferase
- GlcNAc:
-
N-acetyl glucosamine
- GnT:
-
N-acetyl glucosaminyl transferase
- HIV:
-
human immunodeficiency virus
- IFN:
-
interferon
- IgG:
-
immunoglobulin
- LAMP:
-
lysosomal membrane glycoprotein
- ManNAc:
-
N-acetyl mannosamine
- mPL-I:
-
mouse placental lactogen I
- NANA:
-
N-acetyl-neuraminic acid
- NGNA:
-
N-glycolyl-neuraminic acid
- OST:
-
oligosaccharyltransferase
- ST:
-
sialyl transferase
- ST3Gal1:
-
sialyl transferase 3 (CMP-sialic acid: Galβ1,3GalNAc2,3 sialyl transferase)
- TIMP:
-
tissue inhibitors of metalloproteinases
- t-PA:
-
tissue plasminogen activator
- UDP:
-
uridine diphosphate
- UTP:
-
uridine triphosphate
References
Allen S., Naim H.Y. and Bulleid N.J. (1995). Intracellular folding of tissue-type plasminogen activator. Effects of disulfide bond formation on N-linked glycosylation and secretion. J. Biol. Chem. 270: 4797–4804
Andersen D.C. (2004). Cell Culture Effects on the Glycosylation of Therapeutic Proteins. Bioprocess International. IBC Life Sciences, Boston
Andersen D.C., Bridges T., Gawlitzek M. and Hoy C. (2000). Multiple cell culture factors can affect the glycosylation of Asn-184 in CHO-produced tissue-type plasminogen activator. Biotechnol. Bioeng. 70: 25–31
Andersen D.C. and Goochee C.F. (1994). The effect of cell-culture conditions on the oligosaccharide structures of secreted glycoproteins. Curr. Opin. Biotechnol. 5: 546–549
Andersen D.C. and Goochee C.F. (1995). The effect of ammonia on the O-linked glycosylation of granulocyte colony-stimulating factor produced by Chinese hamster ovary cells. Biotechnol. Bioeng. 47: 96–105
Andersen D.C. and Krummen L. (2002). Recombinant protein expression for therapeutic applications. Curr. Opin. Biotechnol. 13: 117–123
Angata T. and Varki A. (2002). Chemical diversity in the sialic acids and related α-keto acids: an evolutionary perspective. Chem. Rev. 102: 439–469
Apweiler R., Hermjakob H. and Sharon N. (1999). On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys. Acta. 1473: 4–8
Backstrom M., Link T., Olson F.J., Karlsson H., Graham R., Picco G., Burchell J., Taylor-Papadimitriou J., Noll T. and Hansson G.C. (2003). Recombinant MUC1 mucin with a breast cancer-like O-glycosylation produced in large amounts in Chinese-hamster ovary cells. Biochem. J. 376: 677–686
Baker K.N., Rendall M.H., Hills A.E., Hoare M., Freedman R.B. and James D.C. (2001). Metabolic control of recombinant protein N-glycan processing in NS0 and CHO cells. Biotechnol. Bioeng. 73: 188–202
Barasch J., Kiss B., Prince A., Saiman L., Gruenert D. and al-Awqati Q. (1991). Defective acidification of intracellular organelles in cystic fibrosis. Nature 352(6330): 70–73
Borys M.C., Linzer D.I. and Papoutsakis E.T. (1993). Culture pH affects expression rates and glycosylation of recombinant mouse placental lactogen proteins by Chinese hamster ovary (CHO) cells. Biotechnology (NY) 11: 720–724
Bragonzi A., Distefano G., Buckberry L.D., Acerbis G., Foglieni C., Lamotte D., Campi G., Marc A., Soria M.R., Jenkins N. and Monaco L. (2000). A new Chinese hamster ovary cell line expressing alpha2,6-sialyltransferase used as universal host for the production of human-like sialylated recombinant glycoproteins. Biochim. Biophys. Acta. 1474(3): 273–282
Breton C., Oriol R. and Imberty A. (1998). Conserved structural features in eukaryotic and prokaryotic fucosyltransferases. Glycobiology 8: 87–94
Butler M. (2004). Animal Cell Culture and Technology. Bios Scientific, Oxford
Butler M., Quelhas D., Critchley A.J., Carchon H., Hebestreit H.F., Hibbert R.G., Vilarinho L., Teles E., Matthijs G., Schollen Argibay P., Harvey D.J., Dwek R.A., Jaeken J. and Rudd P.M. (2003). Detailed glycan analysis of serum glycoproteins of patients with congenital disorders of glycosylation indicates the specific enzyme defect andcoupled with proteomics, provides an insight into pathogenesis. Glycobiology 13: 601–622
Butler M. and Spier R.E. (1984). The effects of glutamine utilisation and ammonia production on the growth of BHK cells in microcarrier cultures. J. Biotechnol. 1: 187–196
Chee Furng Wong D., Tin Kam Wong K., Tang Goh L., Kiat Heng C. and Gek Sim Yap M. (2005). Impact of dynamic online fed-batch strategies on metabolismproductivity and N-glycosylation quality in CHO cell cultures. Biotechnol. Bioeng. 89: 164–177
Chotigeat W., Watanapokasin Y., Mahler S. and Gray P.P. (1994). Role of environmental conditions on the expression levels, glycoform pattern and levels of sialyltransferase for hFSH produced by recombinant CHO cells. Cytotechnology 15: 217–221
Clark K.J., Griffiths J., Bailey K.M. and Harcum S.W. (2005). Gene-expression profiles for five key glycosylation genes for galactose-fed CHO cells expressing recombinant IL-4/13 cytokine trap. Biotechnol. Bioeng. 90: 568–577
Curling E.M., Hayter P.M., Baines A.J., Bull A.T., Gull K., Strange P.G. and Jenkins N. (1990). Recombinant human interferon-gamma. Differences in glycosylation and proteolytic processing lead to heterogeneity in batch culture. Biochem. J. 272: 333–337
Davidson S.K. and Hunt L.A. (1985). Sindbis virus glycoproteins are abnormally glycosylated in Chinese hamster ovary cells deprived of glucose. J. Gen. Virol. 66: 1457–1468
Davies J., Jiang L., Pan L.Z., LaBarre M.J., Anderson D. and Reff M. (2001). Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: Expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for FC gamma RIII. Biotechnol. Bioeng. 74: 288–294
Dawson G., Moskal J.R. and Dawson S.A. (2004). Transfection of 2,6 and 2,3-sialyltransferase genes and GlcNAc-transferase genes into human glioma cell line U-373 MG affects glycoconjugate expression and enhances cell death. J. Neurochem. 89(6): 1436–1444
Doyle C. and Butler M. (1990). The effect of pH on the toxicity of ammonia to a murine hybridoma. J. Biotechnol. 15: 91–100
Egrie J.C., Dwyer E., Browne J.K., Hitz A. and Lykos M.A. (2003). Darbepoetin alfa has a longer circulating half-life and greater in vivo potency than recombinant human erythropoietin. Exp. Hematol. 31: 290–299
Ellgaard L. and Helenius A. (2003). Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 4(3): 181–191
Erbayraktar S., Grasso G., Sfacteria A., Xie Q.W., Coleman T., Kreilgaard M., Torup L., Sager T., Erbayraktar Z., Gokmen N., Yilmaz O., Ghezzi P., Villa P., Fratelli M., Casagrande S., Leist M., Helboe L., Gerwein J., Christensen S., Geist M.A., Pedersen L.O., Cerami-Hand C., Wuerth J.P., Cerami A. and Brines M. (2003). Asialoerythropoietin is a nonerythropoietic cytokine with broad neuroprotective activity in vivo. Proc. Natl. Acad. Sci. U S A 100: 6741–6746
Follstad B.D. 2004. A Method for Increasing Glycoprotein Sialylation in Mammalian Cells. Cell Culture Engineering IX Poster.
Freeze H.H.J. (2002). Sweet solution: sugars to the rescue. Cell Biol. 158: 615–616
Gavel Y. (1990). Sequence differences between glycosylated and non-glycosylated Asn-X-Thr/Ser acceptor sites: implications for protein engineering. Protein Eng. 3(5): 433–442
Gawlitzek M., Valley U., Nimtz M., Wagner R. and Conradt H.S. (1995). Characterization of changes in the glycosylation pattern of recombinant proteins from BHK-21 cells due to different culture conditions. J. Biotech. 42: 117–131
Gawlitzek M., Valley U. and Wagner R. (1998). Ammonium ion and glucosamine dependent increases of oligosaccharide complexity in recombinant glycoproteins secreted from cultivated BHK-21 cells. Biotechnol. Bioeng. 57: 518–528
Goochee C.F. 1992. Bioprocess factors affecting glycoprotein oligosaccharide structure. Dev. Biol. Stand. 76: 95–104.Review.
Goto M., Akai K., Murakami A., Hashimoto C., Tsuda E., Ueda M., Kawanishi G., Takahashi N., Ishimoto A., Chiba H. and Sasaki R. (1988). Production of recombinant human erythropoietin in mammalian cells: Host–cell dependency of the biological activity of the cloned glycoprotein. Bio/Technology 6: 67–71
Grabenhorst E., Hoffmann A., Nimtz M., Zettlmeissl G. and Conradt H.S. (1995). Construction of stable BHK-21 cells coexpressing human secretory glycoprotein and human Gal (β1–4)GlcNAc-R α2,6-sialyltransferase. Eur. J. Biochem. 232: 718–725
Grammatikos S.I., Valley U., Nimtz M., Conradt H.S. and Wagner R. (1998). Intracellular UDP-N-acetylhexosamine pool affects N-glycan complexity: a mechanism of ammonium action on protein glycosylation. Biotechnol. Prog. 14: 410–419
Gu X. and Wang D.I. (1998). Improvement of interferon-gamma sialylation in Chinese hamster ovary cell culture by feeding of N-acetylmannosamine. Biotechnol. Bioeng. 58(6): 642–648
Hayter P.M., Curling E.M.A., Baines A.J., Jenkins N., Salmon I., Strange P.G., Tong J.M. and Bull A.T. (1992). Glucose-limited chemostat culture of chinese hamster ovary cells producing recombinant human interferon-γ. Biotechnol. Bioeng. 39: 327–335
Heidemann R., Lutkemeyer D., Buntemeyer H. and Lehmann J. (1998). Effects of dissolved oxygen levels and the role of extra- and intracellular amino acid concentration upon the metabolism of mammalian cell lines during batch and continuous cultures. Cytotechnology 26: 185–197
Hirschberg C.B. (2001). Golgi nucleotide sugar transport and leukocyte adhesion deficiency II. J. Clin. Invest. 108: 3–6
Hokke C.H., Bergwerff A.A., Kamerling J.P., Vliegenthart J.F. and Dedem G.W. (1995). Structural analysis of the sialylated N- and O-linked carbohydrate chains of recombinant human erythropoietin expressed in Chinese hamster ovary cells. Sialylation patterns and branch location of dimeric N-acetyllactosamine units. Eur. J. Biochem. 228: 981–1008
Jan D.C., Petch D.A., Huzel N. and Butler M. (1997). The effect of dissolved oxygen on the metabolic profile of a murine hybridoma grown in serum-free medium in continuous cultures. Biotech. Bioeng. 54: 153–164
Jenkins N. and Curling E.M. (1994). Glycosylation of recombinant proteins: problems and prospects. Enzyme Microb. Technol. 16: 354–364
Jenkins N., Parekh R.B. and James D.C. (1996). Getting the glycosylation right: implications for the biotechnology industry. Nat. Biotechnol. 14: 975–981
Jones M.B., Teng H., Rhee J.K., Lahar N., Baskaran G. and Yarema K.J. (2004). Characterization of the cellular uptake and metabolic conversion of acetylated N-acetylmannosamine (ManNAc) analogues to sialic acids. Biotechnol. Bioeng. 85(4): 394–405
Kagawa Y., Takasaki S., Utsumi J., Hosoi K., Shimizu H., Kochibe N. and Kobata A. (1988). Comparative study of the asparagine-linked sugar chains of natural human interferon-beta 1 and recombinant human interferon-beta 1 produced by three different mammalian cells. J. Biol. Chem. 263: 17508–17515
Kornfeld R. and Kornfeld S. (1985). Assembly of asparagine-linked oligosaccharides. Ann. Rev. Biochem. 54: 631–664
Kunkel J.P., Jan D.C., Jamieson J.C. and Butler M. (1998). Dissolved oxygen concentration in serum-free continuous culture affects N-linked glycosylation of a monoclonal antibody. J. Biotechnol. 62: 55–71
Kunkel J.P., Yan W.Y., Butler M. and Jamieson J.C. 2003. Decreased monoclonal IgG1 galactosylation at reduced dissolved oxygen concentration is not a result of lowered galactosyltransferase activity in vitro. Society for Glycobiology Meeting, San Diego, CA, Glycobiology 13: 875.
Leonard C.K., Spellman M.W., Riddle L., Harris R.J., Thomas J.N. and Gregory T.J. (1990). Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J. Biol. Chem. 265: 10373–10382
Longmore G.D. and Schachter H. (1982). Product-identification and substrate-specificity studies of the GDP-l-fucose:2-acetamido-2-deoxy-beta-d-glucoside (FUC goes to Asn-linked GlcNAc) 6-alpha-l-fucosyltransferase in a Golgi-rich fraction from porcine liver. Carbohydr. Res. 100: 365–392
Meynial-Salles I. and Combes D. (1996). J. Biotechnol. 46: 1–14
Miyoshi E., Noda K., Ko J.H., Ekuni A., Kitada T., Uozumi N., Ikeda Y., Matsuura N., Sasaki Y., Hayashi N., Hori M. and Taniguchi N. (1999). Overexpression of alpha 1–6 fucosyltransferase in hepatoma cells suppresses intrahepatic metastasis after splenic injection in athymic mice. Cancer Res. 59: 2237–2243
Nabi I.R. and Dennis J.W. (1998). The extent of polylactosamine glycosylation of MDCK LAMP-2 is determined by its Golgi residence time. Glycobiology 8: 947–953
Narhi L.O., Arakawa T., Aoki K.H., Elmore R., Rohde M.F., Boone T. and Strickland T.W. (1991). The effect of carbohydrate on the structure and stability of erythropoietin. J. Biol. Chem. 266: 23022–23026
Noda K., Miyoshi E., Uozumi N., Gao C.X., Suzuki K., Hayashi N., Hori M. and Taniguchi N. (1998). High expression of alpha-1–6 fucosyltransferase during rat hepatocarcinogenesis. Int. J. Cancer. 75: 444–450
Nyberg G.B., Balcarcel R.R., Follstad B.D., Stephanopoulos G. and Wang D.I. (1999). Metabolic effects on recombinant interferon-gamma glycosylation in continuous culture of Chinese hamster ovary cells. Biotechnol. Bioeng. 62: 336–347
Ohyama C., Smith P.L., Angata K., Fukuda M.N., Lowe J.B. and Fukuda M. (1998). Molecular cloning and expression of GDP-d-mannose-4,6-dehydratasea key enzyme for fucose metabolism defective in Lec13 cells. J. Biol. Chem. 273: 14582–14587
Okazaki A., Shoji-Hosaka E., Nakamura K., Wakitani M., Uchida K., Kakita S., Tsumoto K., Kumagai I. and Shitara K. (2004). Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and Fcgamma, RIIIa. J. Mol. Biol. 336: 1239–1249
Pels Rijcken W.R., Overdijk B. and Ferwerda W. (1995). The effect of increasing nucleotide-sugar concentrations on the incorporation of sugars into glycoconjugates in rat hepatocytes. Biochem. J. 305: 865–870
Perlman S., Christiansen J., Gram-Nielsen S., Jeppesen C.B., Andersen K.V., Halkier T., Okkels S. and Schambye H.T. (2003). Glycosylation of an N-terminal extension prolongs the half-life and increases the in vivo activity of follicle stimulating hormone. J. Clin. Endocrinol. Metab. 88: 3227–3235
Petrescu A.J., Milac A.L., Petrescu S.M., Dwek R.A. and Wormald M.R. (2004). Statistical analysis of the protein environment of N-glycosylation sites: implications for occupancy, structureand folding. Glycobiology 14: 103–114
Prati E.G., Matasci M., Suter T.B., Dinter A., Sburlati A.R. and Bailey J.E. (2000). Engineering of coordinated up- and down-regulation of two glycosyltransferases of the O-glycosylation pathway in Chinese hamster ovary (CHO) cells. Biotechnol. Bioeng. 68(3): 239–244
Rademacher T.W., Jaques A. and Williams P.J. (1996). The defining characteristics of immunoglobulin glycosylation. In: Isenberg, D.A. and Rademacher, T.W. (eds) Abnormalities of IgG Glycosylation and Immunological Disorders, pp 1–44. publ. Wiley, NY
Raju T.S., Briggs J.B., Borge S.M. and Jones A.J. (2000). Species-specific variation in glycosylation of IgG: evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiology 10: 477–486
Raju T.S., Briggs J.B., Chamow S.M., Winkler M.E. and Jones A.J. (2001). Glycoengineering of therapeutic glycoproteins: in vitro galactosylation and sialylation of glycoproteins with terminal N-acetylglucosamine and galactose residues. Biochemistry 40: 8868–8876
Rearick J.I., Chapman A. and Kornfeld S. (1981). Glucose starvation alters lipid-linked oligosaccharide biosynthesis in Chinese hamster ovary cells. J. Biol. Chem. 256: 6255–6261
Restelli V. and Butler M. (2002). The effect of cell culture parameters on protein glycosylation. In: Al-Rubeai, M (eds) Glycosylation, vol 3, pp 61–92. Kluwer, Dordrecht
Reuter G. and Gabius H.J. 1999. Eukaryotic glycosylation: whim of nature or multipurpose tool? Cell Mol. Life Sci. 55: 368–422.Review.
Rijcken P.W.R., Overdijk B. and Ferwerda W. (1995). The effect of increasing nucleotide-sugar concentrations on the incorporation of sugars into glycoconjugates in rat hepatocytes. Biochem. J. 305: 865–870
Rothman R.J., Warren L., Vliegenthart J.F. and Hard K.J. (1989). Clonal analysis of the glycosylation of immunoglobulin G secreted by murine hybridomas. Biochemistry 28: 1377–1384
Rudd P.M. and Dwek R.A. (1997). Glycosylation: heterogeneity and the 3D structure of proteins. Critical Rev. Biochem. Mol. Biol. 32: 1–100
Saitoh A., Aoyagi Y. and Asakura H. (1993). Structural analysis on the sugar chains of human alpha 1-antitrypsin: presence of fucosylated biantennary glycan in hepatocellular carcinoma. Arch. Biochem. Biophys. 303: 281–287
Sburlati A.R., Umana P., Prati E.G. and Bailey J.E. (1998). Synthesis of bisected glycoforms of recombinant IFN-beta by overexpression of beta-1,4-N-acetylglucosaminyltransferase III in Chinese hamster ovary cells. Biotechnol. Prog. 14(2): 189–192
Schweikart F., Jones R., Jaton J.C. and Hughes G.J. (1999). Rapid structural characterisation of a murine monoclonal IgA alpha chain: heterogeneity in the oligosaccharide structures at a specific site in samples produced in different bioreactor systems. J. Biotechnol. 69: 191–201
Sears P. and Wong C.H.. (1998). Enzyme action in glycoprotein synthesis. Cell. Mol. Life Sci. 54: 223–252
Seppala R., Lehto V.P. and Gahl W.A. (1999). Mutations in the human UDP-N-acetylglucosamine 2-epimerase gene define the disease sialuria and the allosteric site of the enzyme. Am. J. Hum. Genet. 64: 1563–1569
Sheeley D.M., Merrill B.M. and Taylor L.C. (1997). Characterization of monoclonal antibody glycosylation: comparison of expression systems and identification of terminal alpha-linked galactose. Anal. Biochem. 247: 102–110
Shelikoff M., Sinskey A.J. and Stephanopoulos G. (1994). The effect of protein synthesis inhibitors on the glycosylation site occupancy of recombinant human prolactin. Cytotechnology 15: 195–208
Shields R.L., Lai J., Keck R., Oȁ9Connell L.Y., Hong K., Meng Y.G., Weikert S.H. and Presta L.G. (2002). Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277: 26733–26740
Shinkawa T., Nakamura K., Yamane N., Shoji-Hosaka E., Kanda Y., Sakurada M., Uchida K., Anazawa H., Satoh M., Yamasaki M., Hanai N. and Shitara K. (2003). The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 278: 3466–3473
Shriver Z., Raguram S. and Sasisekharan R. (2004). Glycomics: a pathway to a class of new and improved therapeutics. Nat. Rev. Drug Discov. 3: 863–873
Spellman M.W. (1990). Carbohydrate characterization of recombinant glycoproteins of pharmaceutical interest. Anal. Chem. 62: 1714–1722
Srikrishna G., Varki N.M., Newell P.C., Varki A. and Freeze H.H. (1997). An IgG monoclonal antibody against Dictyostelium discoideum glycoproteins specifically recognizes Fucalpha1,6GlcNAcbeta in the core of N-linked glycans. Localized expression of core-fucosylated glycoconjugates in human tissues. J. Biol. Chem. 272: 25743–25752
Stark N.J. and Heath E.C. (1979). Glucose-dependent glycosylation of secretory glycoprotein in mouse myeloma cells. Arch. Biochem. Biophys. 192: 599–609
Storring P.L. (1992). Assaying glycoprotein hormones–the influence of glycosylation on immunoreactivity. Trends Biotechnol. 10: 427–432
Stubbs H.J., Lih J.J., Gustafson T.L. and Rice K.G. (1996). Influence of core fucosylation on the flexibility of a biantennary N-linked oligosaccharide. Biochemistry 35: 937–947
Sturla L., Etzioni A., Bisso A., Zanardi D., Silengo L. and Tonetti M. (1998). Defective intracellular activity of GDP-d-mannose-4,6-dehydratase in leukocyte adhesion deficiency type II syndrome. FEBS Lett. 429: 274–278
Tachibana H., Kim J. and Shivahata S. (1997). Building high affinity human antibodies by altering the glycosylation on the light chain variable region in N-acetyl glucosamine-supplemented hybridoms cultures. Cytotechnology 23: 151–159
Takahashi T., Ikeda Y., Miyoshi E., Yaginuma Y., Ishikawa M. and Taniguchi N. (2000). Alpha1,6fucosyltransferase is highly and specifically expressed in human ovarian serous adenocarcinomas. Int. J. Cancer. 88: 914–919
Takeuchi M., Takasaki S., Miyazaki H., Kato T., Hoshi S., Kochibe N. and Kobata A. (1988). Comparative study of the asparagine-linked sugar chains of human erythropoietins purified from urine and the culture medium of recombinant Chinese hamster ovary cells. J. Biol. Chem. 263: 3657–3663
Thotakura N.R., Desai R.K., Bates L.G., Cole E.S., Pratt B.M. and Weintraub B.D. (1991). Biological activity and metabolic clearance of a recombinant human thyrotropin produced in Chinese hamster ovary cells. Endocrinology 128: 341–348
Tonetti M., Sturla L., Bisso A. and Benatti U. (1996). Synthesis of GDP-l-fucose by the human FX protein. J. Biol. Chem. 271: 27274–27279
Umana P. and Bailey J.E. (1997). A mathematical model of N-linked glycoform biosynthesis. Biotechnol. Bioeng. 55: 890–908
Umana P., Jean-Mairet J., Moudry R., Amstutz H. and Bailey J.E. (1999). Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat. Biotechnol. 17: 176–180
Uozumi N., Yanagidani S., Miyoshi E., Ihara Y., Sakuma T., Gao C.X., Teshima T., Fujii S., Shiba T. and Taniguchi N. (1996). Purification and cDNA cloning of porcine brain GDP-L-Fuc:N-acetyl-beta-d-glucosaminide alpha 1–6 fucosyltransferase. J. Biol. Chem. 271: 27810–27817
Valley U., Nimtz M., Conradt H.S. and Wagner R. (1999). Incorporation of ammonium into intracellular UDP-activated N-acetylhexosamines and into carbohydrate structures in glycoproteins. Biotechnol. Bioeng. 64(4): 401–417
Rudd P.M., Dwek R.A. and Opdenakker G. (1998). Concepts and principles of O-linked glycosylation. Crit. Rev. Biochem. Mol. Biol. 33(3): 151–208
Voynow J.A., Kaiser R.S., Scanlin T.F. and Glick M.C. (1991). Purification and characterization of GDP-l-fucose-N-acetyl beta-d-glucosaminide alpha 1–6 fucosyltransferase from cultured human skin fibroblasts. Requirement of a specific biantennary oligosaccharide as substrate. J. Biol. Chem. 266: 21572–21577
Walmsley A.R. and Hooper N.M. (2003). Glycosylation efficiency of Asn-Xaa-Thr sequons is independent of distance from the C-terminus in membrane dipeptidase. Glycobiology 13(9): 641–646
Wang W.C., Lee N., Aoki D., Fukuda M.N. and Fukuda M. (1991). The poly-N-acetyllactosamines attached to lysosomal membrane glycoproteins are increased by the prolonged association with the Golgi complex. J. Biol. Chem. 266: 23185–23190
Wang W., Li W., Ikeda Y., Miyagawa J.I., Taniguchi M., Miyoshi E., Sheng Y., Ekuni A., Ko J.H., Yamamoto Y., Sugimoto T., Yamashita S., Matsuzawa Y., Grabowski G.A., Honke K. and Taniguchi N. (2001). Ectopic expression of alpha1,6 fucosyltransferase in mice causes steatosis in the liver and kidney accompanied by a modification of lysosomal acid lipase. Glycobiology 11: 165–174
Wasley L.C., Timony G., Murtha P., Stoudemire J., Dorner A.J., Caro J., Krieger M. and Kaufman R.J. (1991). Blood 77: 2624–2632
Weikert S., Papac D., Briggs J., Cowfer D., Tom S., Gawlitzek M., Lofgren J., Mehta S., Chisholm V., Modi N., Eppler S., Carroll K., Chamow S., Peers D., Berman P. and Krummen L. (1999). Engineering Chinese hamster ovary cells to maximize sialic acid content of recombinant glycoproteins. Nat. Biotechnol. 17: 1116–1121
Weiss P. and Ashwell G. (1989). The asialoglycoprotein receptor: properties and modulation by ligand. Prog. Clin. Biol. Res. 300: 169–184
Wormald M.R., Rudd P.M., Harvey D.J., Chang S.C., Scragg I.G. and Dwek R.A. (1997). Variations in oligosaccharide-protein interactions in immunoglobulin G determine the site-specific glycosylation profiles and modulate the dynamic motion of the Fc oligosaccharides. Biochemistry 36: 1370–1380
Xie L. and Wang D.I. (1997). Integrated approaches to the design of media and feeding strategies for fed-batch cultures of animal cells. Trends Biotechnol. 15: 109–113
Yamashita K., Koide N., Endo T., Iwaki Y. and Kobata A. (1989). Altered glycosylation of serum transferring of patients with hepatocellular carcinoma. J. Biol. Chem. 264: 2415–2423
Yan A. and Lennarz W.J. (2005). Unraveling the mechanism of protein N-glycosylation. J. Biol. Chem. 280: 3121–3124
Yanagidani S., Uozumi N., Ihara Y., Miyoshi E., Yamaguchi N. and Taniguchi N. (1997). Purification and cDNA cloning of GDP-l-Fuc:N-acetyl-beta-d-glucosaminide:alpha1–6 fucosyltransferase (alpha1–6 FucT) from human gastric cancer MKN45 cells. J. Biochem. 121: 626–632
Yang M. and Butler M. (2000). Effects of ammonia on CHO cell growtherythropoietin production and glycosylation. Biotechnol. Bioeng. 68: 370–380
Zanghi J.A., Mendoza T.P., Knop R.H. and Miller W.M. (1998). Ammonia inhibits neural cell adhesion molecule polysialylation in Chinese hamster ovary and small cell lung cancer cells. J. Cell Physiol. 177: 248–263
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Butler, M. Optimisation of the Cellular Metabolism of Glycosylation for Recombinant Proteins Produced by Mammalian Cell Systems. Cytotechnology 50, 57–76 (2006). https://doi.org/10.1007/s10616-005-4537-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-005-4537-x