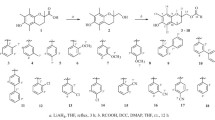
Sixteen tyrosol derivatives were synthesized and characterized by NMR and HR-MS. The antioxidant activity of those compounds was evaluated using four different assays. The results showed that some target compounds displayed better antioxidant activity than L-ascorbic acid and Trolox. Five target compounds exhibited more potent α-glucosidase inhibition activity (18.1–56.7 μM) than acarbose (60.9 μM). Eight target compounds showed some anticholinesterase activities.
Similar content being viewed by others
References
E. Tripoli, M. Giammanco, G. Tabacchi, D. Di Majo, S. Giammanco, and M. La Guardia, Nutr. Res. Rev., 18, 98 (2005).
S. Martin-Pelaez, M. I. Covas, M. Fito, A. Kusar, and I. Pravst, Mol. Nutr. Food. Res., 57, 760 (2013).
E. Escrich, M. Solanas, and R. Moral, Cancer Treat. Res., 159, 289 (2014).
M. Servili, S. Esposto, R. Fabiani, S. Urbani, A. Taticchi, F. Mariucci, R. Selvaggini, and G. F. Montedoro, Inflammopharmacology, 17,76 (2009).
C. Giovannini, E. Straface, D. Modesti, E. Coni, A. Cantafora, M. De Vincenzi, W. Malorni, and R. Masella, J. Nutr., 129, 1269 (1999).
S. Salucci, S. Burattini, M. Battistelli, F. Buontempo, B. Canonico, A. M. Martelli, S. Papa, and E. Falcieri, J. Dermatol. Sci., 80, 61 (2015).
C. St-Laurent-Thibault, M. Arseneault, F. Longpre, and C. Ramassamy, Curr. Alzheimer. Res., 8, 543 (2011).
P. Dewapriya, S. W. Himaya, Y. X. Li, and S. K. Kim, Food. Chem., 141, 1147 (2013).
A. Canuelo, B. Gilbert-Lopez, P. Pacheco-Linan, E. Martinez-Lara, E. Siles, and A. Miranda-Vizuete, Mech. Ageing. Dev., 133, 563 (2012).
L. Sun, H. Fan, L. Yang, L. Shi, and Y. Liu, Molecules, 20, 3758 (2015).
E. Y. Ahn, Y. Jiang, Y. Zhang, E. M. Son, S. You, S. W. Kang, J. S. Park, J. H. Jung, B. J. Lee, and D. K. Kim, Oncol. Rep., 19, 527 (2008).
H. Zang, P. Shen, E. P. Wang, Q. Xu, L. Y. Zhang, G. Q. Xia, J. Y. Zhu, H. Zhang, and X. H. Yang, Chem. J. Chin. Univ., 39, 64 (2018).
G. Appendino, A. Minassi, N. Daddario, F. Bianchi, and G. C. Tron, Org. Lett., 4, 3839 (2002).
T. P. Devasagayam, J. C. Tilak, K. K. Boloor, K. S. Sane, S. S. Ghaskadbi, and R. D. Lele, J. Assoc. Physicians India, 52, 794 (2004).
R. Singh, S. Devi, and R. Gollen, Diabetes. Metab. Res. Rev., 31, 113 (2015).
D. S. Malar and K. P. Devi, Curr. Pharm. Biotechnol., 15, 330 (2014).
S. P. Shah and J. E. Duda, Med. Hypotheses., 85, 1002 (2015).
O. P. Sharma and T. K. Bhat, Food. Chem., 113, 1202 (2009).
L. M. Dong, X. C. Jia, Q. W. Luo, Q. Zhang, B. Luo, W. B. Liu, X. Zhang, Q. L. Xu, and J. W. Tan, Molecules, 22, E1140 (2017).
Q. Wang, D. Zhou, Y. Chen, F. Guan, M. Yin, F. Liu, and Y. Shan, Chem. Nat. Compd., 54, 354 (2018).
Z. Guo, D. Lin, J. Guo, and Y. Zhang, Molecules, 22, E482 (2017).
T. Yuan, C. Wan, K. Liu, and N. P. Seeram, Tetrahedron, 68, 959 (2012).
M. Ozturk, U. Kolak, G. Topcu, S. Oksuz, and M. I. Choudhary, Food. Chem., 126, 31 (2011).
Acknowledgment
This work was supported by the Science and Technology Development Funds of Jilin Province (No. 20160520044JH) and the Science and Technology Projects of Administration of Traditional Chinese Medicine of Jilin Province (No. 2017110). In addition, Hao Zang and Peng Shen contributed equally to this work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2019, pp. 899–904.
Rights and permissions
About this article
Cite this article
Zang, H., Shen, P., Xu, Q. et al. Synthesis and Biological Activities of Tyrosol Phenolic Acid Ester Derivatives. Chem Nat Compd 55, 1043–1049 (2019). https://doi.org/10.1007/s10600-019-02889-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-019-02889-z