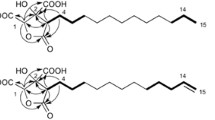
Four verrucosidin derivatives, penicyrone (1), norpenicyrone (2), methyl norpenicyrone (3), and methyl penicyrone (4) were isolated from the hydrothermal vent sulfur-derived fungus Penicillium sp. Y-50-10. Compounds 1–4 were obtained as a mixture of two epimers. Compounds 2–4 have been proved to be new secondary metabolites. The chemical structures were determined by comparing with literature data and HR-MS and 2D NMR spectroscopic analysis. The migration of the double bond from C8=C9 to C7=C8 in compounds 1–4 allowed the assignment of a rearranged verrucosidin skeleton. Compounds 1–4 showed activity against B. subtilis, with MIC values of 32 μg/mL.

Similar content being viewed by others
References
J. W. Blunt, B. R. Copp, R. A. Keyzers, M. H. Munro, and M. R. Prinsep, Nat. Prod. Rep., 32, 116 (2015).
M. Saleem, M. S. Ali, S. Hussain, A. Jabbar, M. Ashraf, and Y. S. Lee, Nat. Prod. Rep., 24, 1142 (2007).
D. Skropeta and L. Wei, Nat. Prod. Rep., 31, 999 (2014).
R. K. Pettit, Mar. Biotech., 13, 1 (2011).
F. Song, H. Dai, Y. Tong, B. Ren, C. Chen, N. Sun, X. Liu, J. Bian, M. Liu, H. Gao, H. Liu, X. Chen, and L. Zhang, J. Nat. Prod., 73, 806 (2010).
L. Zhang, R. An, J. Wang, N. Sun, S. Zhang, J. Hu, and J. Kuai, Curr. Opin. Microbiol., 8, 276 (2005).
M. Ganguli, L.T. Burka, and T. M. Harris, J. Org. Chem., 49, 3762 (1984).
K. Whang, R. J. Cooke, G. Okay, and J. K. Cha, J. Am. Chem. Soc., 112, 8989 (1990).
B. J. Wilson, C. S. Byerly, and L. T. Burka, J. Am. Vet. Med. Assoc., 179, 480 (1981).
R. P. Hodge, C. M. Harris, and T. M. Harris, J. Nat. Prod., 51, 66 (1988).
S.-J. Choo, H.-R. Park, I.-J. Ryoo, J.-P. Kim, B.-S. Yun, Ch.-J. Kim, K. Shin-ya, and I.-D. Yoo, J. Antibiot., 58, 210 (2005).
C. Pan, Y. Shi, B. N. Auckloo, X. Chen, C. T. A. Chen, X. Tao, and B. Wu, Mar. Drugs, 14, 156 (2016).
Y. Y. Bu, H. Yamazaki, O. Takahashi, R. Kirikoshi, K. Ukai, and M. Namikoshi, J. Antibiot., 69, 57 (2016).
G. Appendino, S. Gibbons, A. Giana, A. Pagani, G. Grassi, M. Stavri, E. Smith, and M. M. Rahman, J. Nat. Prod., 71, 1427 (2008).
Acknowledgment
This work was supported by NSFC (Nos. 81273386 and 81573306).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2018, pp. 211–213.
Rights and permissions
About this article
Cite this article
Pan, C., Shi, Y., Auckloo, B.N. et al. Four Verrucosidin Derivatives Isolated from the Hydrothermal Vent Sulfur-Derived Fungus Penicillium sp. Y-50-10. Chem Nat Compd 54, 253–256 (2018). https://doi.org/10.1007/s10600-018-2316-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-018-2316-0