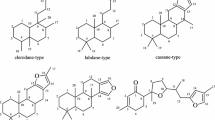
A new quinolizidine alkaloid, (+)-13β-butoxymatrine, was isolated from Oxytropis ochrocephala Bunge. The structure was established by spectroscopic methods, including extensive 1D and 2D NMR experiments.
Similar content being viewed by others
References
Academia Sinica, Flora of China (in Chinese), Vol. 42 (2), Science Press, Beijing, 1998, 21 pp.
H. Lu, S. S. Wang, Q. W. Zhou, Y. N. Zhao, and B. Y. Zhao, Rangeland J., 34, 609 (2012).
R. J. Molyneux and L. F. James, Science, 216, 190 (1982).
G. R. Cao, S. J. Li, D. X. Duan, and X. W. Zhao, J. Northwest Sci-Tech Univ. Agri, 17, 1 (1989).
X. Z. Meng, X. Q. Hu, R. M. Zhang, and Y. T. You, Chin. Trad. Herb. Drug, 25, 61 (1994).
X. Li, S. D. Zhang, H. Z. Jin, F. Dong, L. Shan, and W. D. Zhang, Nat. Prod. Res., 27, 554 (2013).
R. Q. Sun, Z. J. Jia, D. L. Cheng, and Z. Q. Zhu, Planta Med, 58, 211 (1992).
C. J. Tan, L. N. Liu, H. Bi-sihaletu, H. M. Tang, and B. Y. Zhao, Prog. Vet. Med., 36, 71 (2015).
C. J. Tan, L. N. Liu, H. M. Tang, B. Y. Shi, J. Q. Ran, and B. Y. Zhao, Nat. Prod. Res. Dev., 27, 1365 (2015).
L. J. Wu, H. X. Lou, and J. Zhou, Nat. Med. Chem. (in Chinese), 6th edition, People Medical Publishing House, Beijing, 2012, 414 pp.
X. J. Liu, M. A. Cao, W. H. Li, C. S. Shen, S. Q. Yan, and C. S. Yuan, Fitoterapia, 81, 524 (2010).
V. Galasso, F. Asaro, F. Berti, B. Pergolese, B. Kovac, and F. Pichierri, Chem. Phys., 330, 457 (2006).
C. J. Tan, P. Yi, M. Goto, S. L. Morris-Natschke, L. N. Liu, K. H. Lee, and B. Y. Zhao, Helv. Chim. Acta, 99, 225 (2016).
C. J. Tan, Yu. Zhao, M. Goto, K. Y. Hsieh, X. M. Yang, S. L. Morris-Natschke, L. N. Liu, B. Y. Zhao, and K. H. Lee, Bioorg. Med. Chem. Lett., 26, 1495 (2016).
L. N. Liu, J. Q. Ran, L. J. Li, Y. Zhao, M. Goto, Susan L. Morris-Natschke, K. H. Lee, B. Y. Zhao, and C. J. Tan, Tetrahedron Lett., 57, 5047 (2016).
P. Skehan, R. Storeng, D. Scudiero, A. Monks, J. McMahon, D. Vistica, J. T. Warren, H. Bokesch, S. Kenney, and M. R. Boyd, J. Nat. Cancer Inst., 82, 1107 (1990).
Acknowledgment
This work was financially supported by the Special Scientific Research Fund of Agriculture Public Welfare Industry (Grant Nos. 201203062, 31660103), the Chinese National Natural Science Foundation (31360084) and the Natural Science Foundation of Guizhou Province (20102273).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2017, pp. 273–274.
Rights and permissions
About this article
Cite this article
Tan, CJ., Liu, LN. & Zhao, BY. A New Quinolizidine Alkaloid from Oxytropis ochrocephala . Chem Nat Compd 53, 322–324 (2017). https://doi.org/10.1007/s10600-017-1979-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-1979-2