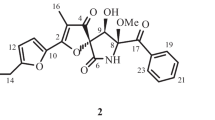
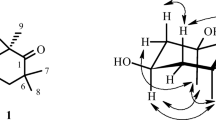
A new fatty acid, (2E,4E,6S)-6-hydroxydeca-2,4-dienoic acid (1), along with six known compounds (2–7), were isolated from the gorgonian-derived fungus Xylaria sp. C-2, collected from the South China Sea. The structure of 1 was elucidated by 1D, 2D NMR, and MS spectrometry. The absolute configuration of 1 was determined by using the modified Mosher’s method. All of the isolated compounds were evaluated for their cytotoxic and antibacterial activities.


Similar content being viewed by others
References
Z. M. Chen, H. B Huang, Y. C. Chen, Z. W. Wang, J. Y. Ma, B. Wang, W. M. Zhang, C. S. Zhang, and J. H. Ju, Helv. Chim. Acta, 94, 1671 (2011).
L. A. McDonald, L. R. Barbieri, V. S. Bernan, J. Janso, P. Lassota, and G. T. Carter, J. Nat. Prod., 67, 1565 (2004).
X. S. Huang, X. F. Sun, S. E. Lin, Z. E. Xiao, H. X. Li, D. Bo, and Z. G. She, Nat. Prod. Res., 28, 111 (2014).
M. Chen, C. L. Shao, C. J. Kong, Z. G. She, and C. Y. Wang, Chem. Nat. Compd., 50, 617 (2014).
M. Chen, C. L. Shao, H. Meng, Z. G. She, and C. Y. Wang, J. Nat. Prod., 77, 2720 (2014).
M. Chen, C. L. Shao, K. L. Wang, Y. Xu, Z. G. She, and C. Y. Wang, Tetrahedron, 70, 9132 (2014).
I. Ohtani, T. Kusumi, Y. Kashman, and H. Kakisawa, J. Am. Chem. Soc., 113, 4092 (1991).
F. J. Arriaga-Giner, E. Wollenweber, I. Schober, and G. Yatskievych, Z. Naturforsch. C, 43, 337 (1988).
C. H. Lu, S. S. Liu, J. Y. Wang, M. Z. Wang, and Y. M. Shen, Helv. Chim. Acta, 97, 334 (2014).
S. C. Hu, R. X. Tan, K. Hong, Z. N. Yu, and H. L. Zhu, Acta Crystallogr. E, 61, 1654 (2005).
T. Okuno, S. Oikawa, T. Goto, K. Sawai, H. Shirahama, and T. Matsumoto, Agric. Biol. Chem., 50, 997 (1986).
D. M. Que, H. F. Dai, Y. B. Zeng, J. Wu, and W. L. Mei, Chin. J. Med. Chem., 3, 200 (2009).
M. Zhang, X. L. Tang, and G. Q. Li, J. Ocean. Univ. China, 5, 89 (2010).
K. Trisuwan, V. Rukachaisirikul, S. Phongpaichit, S. Preedanon, and J. Sakayaroj, Arch. Pharm. Res., 34, 709 (2011).
M. A. M. Mondol, J. H. Kim, M. Lee, F. S. Tareq, H. S. Lee, Y. J. Lee, and H. J. Shin, J. Nat. Prod., 74, 1606 (2011).
G. Appendio, S. Gibbons, A. Giana, A. Pagani, G. Grassi, M. Stavri, E. Smith, and M. M. Rahman, J. Nat. Prod., 71, 1427 (2008).
P. Skehan, R. Storeng, D. Scudiero, A. Monks, J. McMahon, D. Vistica, J. T Warren, H. Bokesch, S. Kenney, and M. R. Boyd, J. Natl. Cancer Inst., 82, 1107 (1990).
Acknowledgment
We thank Dr. Chang-Lun Shao, School of Medicine and Pharmacy, Ocean University of China, for sample collection. This work was supported by the National Natural Science Foundation of China (Nos. 81172977; U1406402) and the Special Financial Fund of Innovative Development of Marine Economic Demonstration Project (No. GD2012-D01-001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2017, pp. 195–198.
Rights and permissions
About this article
Cite this article
Sun, DW., Cao, F., Liu, M. et al. New Fatty Acid From a Gorgonian-Derived Xylaria sp. Fungus. Chem Nat Compd 53, 227–230 (2017). https://doi.org/10.1007/s10600-017-1958-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-1958-7