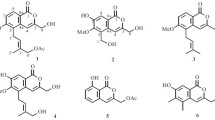
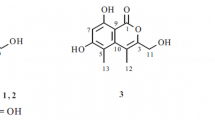
Two new coumarins, 7-(4-hydroxyphenyl)-6H-[1, 3]dioxolo[4,5-g]chromen-6-one (1) and 7-(2-hydroxy-3,4-dimethoxyphenyl)-6H-[1,3]dioxolo[4,5-g]chromen-6-one (2), together with five known coumarins (3–7), were isolated from the roots and stems of flue-cured tobacco (a variety of Nicotiana tabacum L.). Their structures were elucidated by spectroscopic methods, including extensive 1D and 2D NMR techniques. Compounds 1 and 2 were tested for their anti-tobacco mosaic virus (anti-TMV) activity, and they show potential anti-TMV activity with inhibition rates of 14.5 and 31.2%, respectively.

Similar content being viewed by others
References
The Editorial Committee of the Administration Bureau of Flora of China, Flora of China, Vol. 67, Beijing Science and Technology Press, Beijing, 2005.
T. W. Hu and Z. Mao, Tob. Control, 15, i37 (2006).
A. Rodgman and T. A. Perfetti, The Chemical Components of Tobacco and Tobacco Smoke, CRC Press, Taylor and Francis Group, Boca Raton, Florida, 2008.
A. P. Cavender and M. Alban, J. Ethnobiol. Ethnomed., 5, 3 (2009).
A. Inta, P. Shengji, H. Balslev, P. Wangpakapattanawong, and C. Trisonthi, J. Ethnopharmacol., 116, 508 (2008).
X. Feng, J. S. Wang, J. Luo, and L. Y. Kong, J. Asian Nat. Prod. Res., 12, 252 (2010).
Y. Shinozaki, T. Tobita, M. Mizutani, and T. Matsuzaki, Biosci. Biotechnol. Biochem., 60, 903 (1996).
T. Petterson, A. M. Eklund, and I. Wahlberg, J. Agric. Food. Chem., 41, 2097 (1993).
X. C. Wei, S. C. Sumithran, A. G. Deaciuc, H. R. Burton, L. P. Bush, L. P. Dwoskin, and P. A. Crooks, Life Sci., 78, 495 (2005).
T. Braumann, G. Nicolaus, W. Hahn, and H. Elmenhorst, Phytochemistry, 29, 3693 (1990).
Y. K. Chen, X. S. Li, G. Y. Yang, Z. Y. Chen, Q. F. Hu, and M. M. Miao, J. Asian. Nat. Prod. Res., 14, 450 (2012).
X. M. Gao, X. S. Li, X. Z. Yang, H. X. Mu, Y. K. Chen, G. Y. Yang, and Q. F. Hu, Heterocycles, 85, 147 (2012).
Z. Y. Chen, J. L. Tan, G. Y. Yang, M. M. Miao, Z. Y. Chen, and T. F. Li, Phytochem. Lett., 5, 233 (2012).
J. X. Chen, H. Q. Leng, Y. X. Duan, W. Zhao, G. Y. Yang, Y. D. Guo, Y. K. Chen, and Q. F. Hu, Phytochem. Lett., 6, 144 (2013).
J. L. Tan, Z. Y. Chen, G. Y. Yang, M. M. Miao, Y. K. Chen, and T. F. Li, Heterocycles, 83, 2381 (2011).
W. Li, L. B. Zhang, J. H. Peng, N. Li, and X. Y. Zhu, Ind. Crop. Prod., 16, 341 (2008).
D. R. Mou, W. Zhao, T. Zhang, L. Wan, G. Y. Yang, Y. K. Chen, Q. F. Hu, and M. M Miao, Heterocycles, 85, 2485 (2012).
W. H. Zhong, C. J. Zhu, M. Shu, K. D. Sun, L. Zhao, C. Wang, Z. J. Ye, and J. M. Chen, Bioresour. Technol., 101, 6935 (2010).
D. Q. Yu and J. S. Yang, Handbook of Analytical Chemistry, Vol. 7, Nuclear Magnetic Resonance spectroscopy, Chemical Industry Press, 2nd Ed., Beijing, 1999.
A. Hitoshti, K. Ichiro, I. Kazuhiko, and I. Kazuo, J. Nat. Prod., 49, 366 (1986).
J. F. Liu, K. P. Xu, F. S. Li, J. Shen, C. P. Hu, H. Zou, F. Yang, G. R. Liu, H. L. Xiang, Y. J. Zhou, Y. J. Li, and G. S. Tan, Chem. Pharm. Bull., 58, 549 (2010).
Q. F. Hu, B. Zhou, X. M. Gao, L. Y. Yang, L. D. Shu, Y. Q. Shen, G. P. Li, C. T. Che, and G. Y. Yang, J. Nat. Prod., 75, 1909 (2012).
Acknowledgment
This project was supported financially by the Basic Research Foundation of Yunnan Tobacco Industry Co. Ltd. (2012JC01) and the National Natural Science Foundation of China (No. 21002085).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2015, pp. 40–42.
Rights and permissions
About this article
Cite this article
Lei, C., Xu, W., Wu, J. et al. Coumarins from the Roots and Stems of Nicotiana tabacum and their Anti-Tobacco Mosaic Virus Activity. Chem Nat Compd 51, 43–46 (2015). https://doi.org/10.1007/s10600-015-1199-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-015-1199-6