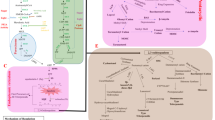
Methods were proposed for preparing 15,16-dihydroisopimaric acid and its methyl ester. Oxidation of methyl dihydroisopimarate through the action of 3-chloroperbenzoic acid occurred with formation of 7,8-epoxy-derivatives and 7α-hydroxydihydrosandaracopimarate. The latter in addition to 7-oxotetrahydroisopimarate were formed through the action of t-butylhydroperoxide in the presence of MoCl5. Oxidation of dihydroisopimaric acid or the ester by selenium dioxide in dioxane gave the corresponding 14α-hydroxy-derivatives. Pyridinium chlorochromate transformed 14α-hydroxydihydroisopimarate into a mixture of methyl esters of 7α-hydroxy-8,14-epoxyisopimaric, 7-oxo-8,14-epoxyisopimaric, 7-oxo-14-hydroxyisopimaric, and 7α-hydroxysandaracopimaric acids. Oximation of methyl 7-oxotetrahydroisopimarate by hydroxylamine hydrochloride in MeOH in the presence of NaOAc gave a mixture of the corresponding (Z)- and (E)-oximes. Beckmann rearrangement of the 7-(E)-oxime of methyl tetrahydroisopimarate through the action of thionylchloride led to tetradecahydrodibenzo[b,d]azepine.
Similar content being viewed by others
References
Yu. V. Kharitonov, E. E. Shul’ts, M. M. Shakirov, M. A. Pokrovskii, A. G. Pokrovskii, and G. A. Tolstikov, Izv. Akad. Nauk, Ser. Khim., 2046 (2013).
A. Nardi, V. Calderone, S. Chericoni, and I. Morelli, Planta Med., 69, 885 (2003).
H. Wulff and B. S. Zhorov, Chem. Rev., 108, 1744 (2008).
Y. Imaizumi, K. Sakamoto, A. Yamada, A. Hotta, S. Ohya, K. Muraki, M. Uchiyama, and T. Ohwada, Mol. Pharmacol., 62, 836 (2002).
G. A. Tolstikov, T. G. Tolstikova, E. E. Shul’ts, S. E. Tolstikov, and M. V. Khvostov, in: Resinous Acids of Russian Conifers [in Russian], B. A. Trofimov (ed.), Geo, Novosibirsk, 2011, 395 pp.
S. B. Singh, M. A. Goetz, D. L. Zink, A. W. Dombrowski, J. D. Polishook, M. L. Garcia, W. Schmalhofer, O. B. McManus, and G. J. Kaczorowski, J. Chem. Soc., Perkin Trans. 1, 3349 (1994).
X. Xia, J. Zhang, Y. Zhang, F. Wei, X. Liu, A. Jia, C. Liu, W. Li, Z. She, and Y. Lin, Bioorg. Med. Chem. Lett., 22, 3017 (2012).
W. Antkowiak, O. E. Edwards, R. Howe, and J. W. ApSimon, Can. J. Chem., 43, 1257 (1965).
B. Papillaud, F. Tiffon, M. Taran, and B. Arreguy-San Miguel, Tetrahedron, 41, 1845 (1985).
Y.-M. Cui, E. Yasutomi, Y. Otani, T. Yoshinaga, K. Ido, K. Sawada, M. Kawahata, K. Yamaguchi, and T. Ohwada, Bioorg. Med. Chem. Lett., 18, 6386 (2008).
T. Tashima, Y. Toriumi, Y. Mochizuki, T. Nonomura, S. Nagaoka, K. Furukawa, H. Tsuru, S. Adachi-Akahane, and T. Ohwada, Bioorg. Med. Chem., 14, 8014 (2006).
J. W. ApSimon, P. V. Demarco, and J. Lemke, Can. J. Chem., 43, 2793 (1965).
A. S. Feliciano, M. Medarde, E. Caballero, F. Tome, and B. Hebrero, J. Chem. Soc., Perkin Trans. 1, 1665 (1992).
W. Herz, D. Melchior, R. N. Mirrington, and P. J. S. Pauwels, J. Org. Chem., 30, 1873 (1965).
Yu. V. Kharitonov, E. E. Shul’ts, Yu. V. Gatilov, I. Yu. Bagryanskaya, M. M. Shakirov, and G. A. Tolstikov, Chem. Nat. Compd., 48, 250 (2012).
H. G. O. Becker, G. Domschke, and E. Fanghanel, Organikum: organisch-chemisches Grundpraktikum [in German], Dt. Verl. der Wiss., Berlin, 1977.
E. J. Corey and J. W. Suggs, Tetrahedron Lett., 2647 (1975).
M. Fieser and L. F. Fieser, Reagents for Organic Synthesis, Vol. 4, John Wiley & Sons, New York, 1974.
G. C. Harris and T. F. Sanderson, J. Am. Chem. Soc., 70, 2079 (1948).
Acknowledgment
The work was supported financially by RFBR grant No. 12-03-00535.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2013, pp. 916–923.
* For No. XXXII, see the literature [1].
Rights and permissions
About this article
Cite this article
Kharitonov, Y.V., Shul’ts, E.E. & Shakirov, M.M. Synthetic Transformations of Higher Terpenoids. XXXIII.* Preparation of 15,16-Dihydroisopimaric Acid and Methyl Dihydroisopimarate and their Transformations. Chem Nat Compd 49, 1067–1075 (2014). https://doi.org/10.1007/s10600-014-0823-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-014-0823-1