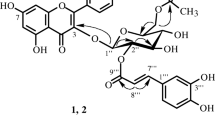
The known flavonoids pinostrobin (5-hydroxy-7-methoxyflavanone), 2',6'-dihydroxy-4',5'-dimethoxychalcone (polygochalcone), astragalin, isoquercitrin, quercitrin, and also the new natural compound 6'-hydroxy2',4'-dimethoxychalcone (persicochalcone) were isolated from the aerial part of lady’s thumb (Polygonum persicaria L.) and characterized using PMR and UV spectroscopy and mass spectrometry.
Similar content being viewed by others
References
State Registry of Drugs [in Russian], Vol. 1, Official Edition, Moscow, 2008, 1238 pp.
V. A. Kurkin, Pharmacognosy [in Russian], 2nd Ed., Revised and Supplemented, Handbook, OOO Ofort, GOU VPO SamGMU, Samara, 2007, 1239 pp.
Plant Resources of Russia: Wild Flowering Plants, Their Component Composition and Biological Activity, Vol. 1, Families Magnoliaceae-Juglandaceae, Ulmaceae, Moraceae, Cannabiaceae, Urticaceae [in Russian], Chief Ed. A. L. Budantsev, Tovarishchestvo Nauchnykh Izdanii KMK, St. Petersburg, Moscow, 2008, 421 pp.
Plant Resources of the USSR. Flowering Plants, Their Chemical Composition and Use. Families Magnoliaceae-Limoniaceae [in Russian], Nauka, Leningrad, 1985, 460 pp.
M. M. Mukhamed'yarova, Khim. Prir. Soedin., 131 (1968).
H. D. Smolarz, Acta Pol. Pharm., 59 (2), 145 (2002).
H. Yoon, S. Eom, J. Hyun, G. Jo, D. Hwang, S. Lee, Y. Yong, J. C. Park, Y. H. Lee, and Y. Lim, Bull. Korean Chem. Soc., 32 (6), 2101 (2011).
J. P. Dzoyem, A. H. L. Nkuete, V. Kuete, M. F. Tala, H. K. Wabo, S. K. Guru, V. S. Rajput, A. Sharma, P. Tane, I. A. Khan, A. K. Saxena, H. Laatsch, and N. H. Tan, Planta Med., 78 (8), 787 (2012).
A. V. Kurkina, T. K. Ryazanova, and V. A. Kurkin, Khim. Prir. Soedin., 717 (2013).
D.-Y. Lee, H.-N. Lyu, H.-Y. Kwak, L. Jung, Y.-H. Lee, D.-K. Kim, I.-S. Chung, S. H. Kim, and N.-I. Baek, J. Appl. Biol. Chem., 50 (3), 144 (2007).
T. Nanamura, T. Hagiwara, and H. Kawagishi, Biosci. Biotechnol. Biochem., 69 (2), 280 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2013, pp. 728–730.
Rights and permissions
About this article
Cite this article
Kurkina, A.V., Ryazanova, T.K. & Kurkin, V.A. Flavonoids from the Aerial Part of Polygonum persicaria . Chem Nat Compd 49, 845–847 (2013). https://doi.org/10.1007/s10600-013-0761-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-013-0761-3