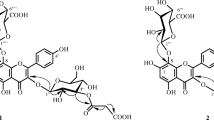
Phytochemical investigation of the aerial parts of Sanchezia nobilis Hook. (Acanthaceae) has yielded matsutake alcohol (1-octen-3-ol) (1) and four matsutake alcohol glycosides identified as 3-O-β-glucopyranosyl-1-octen-3-ol (2), 3-O-β-glucopyranosyl-(1→6)-β-glucopyranosyl-1-octen-3-ol (3), 3-O-α-arabinopyranosyl-(1→6)-β-glucopyranosyl-1-octen-3-ol (4), and 3-O-α-arabinopyranosyl-(1→6)-β-glucopyranosyl-(1→6)-β-glucopyranosyl-1-octen-3-ol (5). The structures of the isolated compounds were assigned on the basis of different techniques of NMR spectral analysis. Compounds 1–4 have been isolated here for the first time from the family Acanthaceae, while compound 5 is isolated here for the first time from a natural source.
Similar content being viewed by others
References
K. R. Kirtikar and B. D. Basu, Indian Medicinal Plants, Jayyed press, Delhi-6, Vol. III, 2nd Ed., 1975.
J. M. Watt and M. G. Breyer-Brandwijk, The Medicinal and Poisonous Plants of Southern and Eastern Africa, 2nd Ed., E. and S. Livingstone Ltd., Edinburgh and London, 1962.
A. E. Abd-Ellah, K. M. Mohamed, E. Y. Bakheet, and H. Mohamed, Bull. Pharm. Sci. Assiut Univ., 29 (II), 300 (2006).
S. Yamamura, K. Ozawa, K. Ohtani, R. Kasai, and K. Yamasaki, Phytochemistry, 48, 131 (1998).
S. Takano, M. Yanase, M. Takahashi, and K. Ogasawara, Chem. Lett., 2017 (1987).
T. Kurihara and M. Kikuchi, Yakugaku Zasshi, 93, 1201 (1973).
S. Murahashi, Chem. Res., 34, 155 (1938).
W. Freytag and K. H. Ney, Eur. J. Biochem., 4, 315 (1968).
E. Honkanen and T. Moisio, Acta. Chim. Scand., 17, 858 (1963).
M. Yoshikawa, T. Murakami, and M. Kubo, Chem. Pharm. Bull., 46 (5), 886 (1998).
M. S. Kamel, K. M. Mohamed, H. A. Hassanean, K. Ohtani, R. Kasai, and K. Yamasaki, Phytochemistry, 55, 252 (2000).
H. Bradbury and J. Jenkins, Carbohydr. Res., 125, 126 (1984).
S. Wang, E. L. Ghisalberti, and J. Ridsdill, J. Nat. Prod., 61 (4), 508 (1998).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2012, pp. 829–831.
Rights and permissions
About this article
Cite this article
Ellah, A.E.A., Mohamed, K.M., Backheet, E.Y. et al. Matsutake alcohol glycosides from Sanchezia nobilis . Chem Nat Compd 48, 930–933 (2013). https://doi.org/10.1007/s10600-013-0431-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-013-0431-5