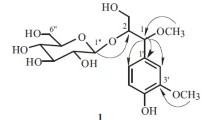
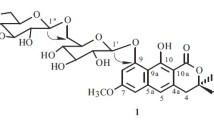
A new furostanol glycoside, named ophiopogonin J (1), was isolated from the fibrous root of Ophiopogon japonicas. The structure of the compound was established as (25R)-26-[(O-β-D-glucopyranosyl-(1 → 2)-β-D-glucopyranosyl)]-20α -hydroxyfurost-5, 22-diene-3-O-α-L-rhamnopyranosyl-(1 → 2)-[β-D-xylopyranosyl(1 → 4)]-β-D-glucopyranoside on the basis of spectroscopic methods, including HR-ESI-MS and 1D and 2D NMR experiments.

Similar content being viewed by others
References
T. M. Loftus, D. E. Jaworsky, G. L. Frehywot, C. A. Townsend, G. V. Ronnett, M. D. Lane, and F. P. Kuhajda, Science, 288, 2379 (2000).
Y. Watanabe, S. Sanada, Y. Ida, and J. Shoji, Chem. Pharm. Bull., 31, 3486 (1983).
Y. Watanabe, Y. Hirai, S. Sanada, and J. Shoji, Shoyakugaku Zasshi, 44, 117 (1990).
A. C. Do, K. Y. Jung, and Y. K. Sung, J. Nat. Prod., 58, 778 (1995).
C. L. Duan, Y. J. Li, P. Li, Y. Jiang, J. X. Liu, and P. F. Tu, Helv. Chim. Acta, 93, 227 (2010).
C. L. Duan, X. F. Ma, Y. Jiang, J. X. Liu, and P. F. Tu, J. Asian Nat. Prod. Res., 12, 745 (2010).
L. P. Kang, Z. J. Liu, D. W. Tan, Y. Zhao, Y. Zhao, H. B. Chen, and B. P. Ma, Magn. Reson. Chem., 45, 725 (2007).
P. K. Agrawal, D. C. Jain, and A. K. Pathak, Magn. Reson. Chem., 33, 923 (1995).
B. Shao, H. Z. Guo, Y. J. Cui, M. Ye, J. Han, and D. Guo, Phytochemistry, 68, 623 (2006).
P. K. Argrawal, Steroids, 70, 715 (2005).
T. Zhang, P. Zou, L. P. Kang, H. S. Yu, Y. S. Liu, X. B. Song, and B. P. Ma, J. Asian Nat. Prod. Res., 11, 824 (2009).
Acknowledgment
This work was supported by Special Science and Technology Projects for Outstanding Young in Life Sciences (KSCX2-EW-Q-19), the Twelfth Five Year Plan Basic Frontier Project in Life Sciences (Y129015EA2), Program for Changjiang Scholar and Innovative Team in University (No. 985-2-063-112), the President Funding of GUCAS (O95101PY00), as well as the K. C. Wong Education Foundation, Hong Kong.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Materials presented at the 9th International Symposium on the Chemistry of Natural Compounds (SCNC, People' s Republic of China, Urumqi, Oct. 16–19, 2011).
Published in Khimiya Prirodnykh Soedinenii, No. 4, July–August, 2012, pp. 551–553.
Rights and permissions
About this article
Cite this article
Duan, C., Wang, Y., Ma, X. et al. A new furostanol glycoside with fatty acid synthase inhibitory activity from Ophiopogon japonicusa . Chem Nat Compd 48, 613–615 (2012). https://doi.org/10.1007/s10600-012-0325-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-012-0325-y