A preparative synthesis of spinochrome E (2,3,5,6,7,8-hexahydroxy-1,4-naphthoquinone, 1), a metabolite of sea urchins of the genus Echinothrix, is proposed starting from 2,3-dichloro-6,7-diethoxynaphthazarine (4) with simultaneous substitution of the Cl atoms by hydroxyl- and nitro-groups, reduction of the latter, and subsequent removal of alkoxy groups and hydrolysis of the amine in the resulting 3-amino-2-hydroxy-6,7-diethoxynaphthazarine (6).
Similar content being viewed by others
Notes
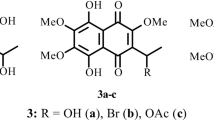
Structures of naphthazarine derivatives are given only for one of all possible tautomers.
References
a) R. H. Thomson, Naturally Occurring Quinones, 2nd Ed., Academic Press, London, New York, 1971; b) 3rd Ed., Chapman & Hall, London, New York, 1987.
M. Service and A. C. Wardlaw, Comp. Biochem. Physiol., 79, 161 (1984); G. Br. Pat. 2,159,056; Chem. Abstr., 104, 83795 (1986); V. P. Anufriev, V. L. Novikov, O. B. Maximov, G. B. Elyakov, D. O. Levitsky, A. V. Lebedev, S. M. Sadretdinov, A. V. Shvilkin, N. I. Afonskaya, M. Ya. Ruda, and N. M. Cherpachenko, Bioorg. Med. Chem. Lett., 8, 587 (1998).
a) G. B. Elyakov, O. B. Maksimov, et al., RF Pat. No. 2,137,472; Byull. Izobret., 26, Chem. Abstr., 132, 284239b (1999); b) G. B. Elyakov, O. B. Maksimov, et al., RF Pat. No. 2,134,107; Byull. Izobret., 22 (1999).
N. P. Mishchenko, S. A. Fedoreev, and V. L. Bagirova, Khim.-farm. Zh., 37, 49 (2003).
I. Singh, R. E. Moore, C. W. J. Chang, R. T. Ogata, and P. J. Scheuer, Tetrahedron, 24, 2969 (1968).
V. F. Anufriev, Dissertation, Pacific Inst. Bioorg. Chem., FEB, RAS, Vladivostok, 1988.
H. Ulrich and R. Richter, in: I. Houben and T. Weyl, Methoden der Organischen Chemie, Georg Thieme Verlag, Stuttgart (1977), Bd. 7/3a.
N. S. Polonik, V. P. Anufriev, and S. G. Polonik, Nat. Prod. Commun., 6, 217 (2011).
J. Smith and R. H. Thomson, Tetrahedron Lett., No. 1, 10 (1960).
G. V. Malinovskaya, A. Ya. Chizhova, and V. F. Anufriev, Izv. Akad. Nauk, Ser. Khim., 1019 (1999) [Russ. Chem. Bull. (Engl. Transl.), 48, 1010 (1999)].
V. P. Anufriev, V. L. Novikov, G. V. Malinovskaya, and V. P. Glazunov, Synth. Commun., 27, 119 (1997).
Acknowledgment
The work was supported financially partially by a grant of the RAS Presidium (Molecular and Cellular Biology Program) and an Interdisciplinary Integrated Project of the Far-East, Siberian, and Ural Branches of the RAS (No. 09-11-SB-05-001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2012, pp. 187–189.
Rights and permissions
About this article
Cite this article
Borisova, K.L., Anufriev, V.F. Simple preparative synthesis of spinochrome e, a pigment from sea urchins of the genus Echinothrix . Chem Nat Compd 48, 202–204 (2012). https://doi.org/10.1007/s10600-012-0204-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-012-0204-6