Abstract
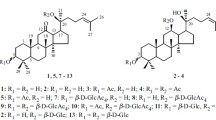
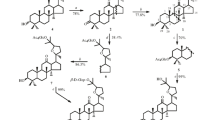
A method for preparative production of 3β,20S-dihydroxydammar-24-en-12-one 3,20-di-O-β-D-glucopyranoside (1), a glycoside from Panax japonicus, chikusetsusaponin-LT8 was developed. Chemical transformation of betulafolientriol, a component of Betula leaves extract, produced the 12-keto-20S-protopanaxadiol (3β,20S-dihydroxydammar-24-en-12-one) (2), exhaustive glycosylation of which by 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosylbromide (3) under Koenigs—Knorr reaction conditions with subsequent removal of protecting groups formed 3β,20S-dihydroxydammar-24-en-12-one 3,20-di-O-β-D-glucopyranoside (1). The principal glycosylation product was 3β,20S-dihydroxydammar-24-en-12-one 3-O-β-D-glucopyranoside if equimolar amounts of (2) and (3) were used.
Similar content being viewed by others
References
S. Yahara, O. Tanaka, and I. Nishioka, Chem. Pharm. Bull., 26, 3010 (1978).
E. Schlosser and G. Wulff, Z. Naturforsch. B: Anorg. Chem. Org. Chem. Biochem. Biophys. Biol., 24, 1284 (1969).
T. Nikaido, T. Ohmoto, U. Sankawa, O. Tanaka, R. Kasai, J. Shoji, S. Sanada, S. Hiai, H. Yokoyama, H. Oura, and Y. Kawashima, Chem. Pharm. Bull., 32, 1477 (1984).
M. Takechi, S. Shimada, and Y. Tanaka, Planta Med., 58, 128 (1992).
Jpn. Pat. No. 8-119866 (1966); Chem. Abstr., 125, 123688n (1996)
L. N. Atopkina, G. V. Malinovskaya, G. B. Elyakov, N. I. Uvarova, H. J. Woerdenbag, A. Koulman, N. Pras, and P. Potier, Planta Med., 65, 30 (1999).
L. N. Atopkina, E. B. Shentsova, M. M. Anisimov, and N. I. Uvarova, Rast. Resur., 36, 89 (2000).
L. N. Atopkina, E. B. Shentsova, M. M. Anisimov, and N. I. Uvarova, Rast. Resur., 37, 76 (2001).
L. N. Atopkina, V. A. Denisenko, N. I. Uvarova, and G. B. Elyakov, Carbohydr. Res., 177, 101 (1988).
L. N. Atopkina, N. I. Uvarova, and G. B. Elyakov, Carbohydr. Res., 303, 449 (1997).
N. K. Kochetkov, ed., Methods of Carbohydrate Chemistry [Russian translation], Mir, Moscow (1967), p. 123.
F. G. Fisher and N. Seiler, Liebigs Ann. Chem., 626, 185 (1959).
Author information
Authors and Affiliations
Additional information
__________
Translated from Khimiya Prirodnykh Soedinenii, No. 1, pp. 44–48, January–February, 2006.
Rights and permissions
About this article
Cite this article
Atopkina, L.N., Denisenko, V.A. Synthesis of 3β,20S-dihydroxydammar-24-en-12-one 3,20-di-O-β-D-glucopyranoside (chikusetsusaponin-LT8), a glycoside from Panax japonicus . Chem Nat Compd 42, 55–60 (2006). https://doi.org/10.1007/s10600-006-0035-4
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10600-006-0035-4