Abstract
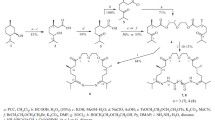
A synthesis of the promising optically pure synthon isopropyl-4R-methyl-6-iodohexanoate based on ozonolytic transformation of the product of regiospecific dehydratation of L-(-)-menthol, (R)-p-menth-3-ene, into 2,6R-dimethyl-8-hydroxyoctan-3-one was proposed.
Similar content being viewed by others
REFERENCES
H. Yoshimoto, Jpn. Pat. No. 01261347 [89, 261, 347] (C1. C07C53/21), 18 Oct. 1989, Appl. 88/88285, 12 Apr. 1988; Chem. Abstr., 112, 197633u (1990).
T. Suguro and K. Mori, Agric. Biol. Chem., 43, 869 (1979).
K. Mori, S. Tamada, T. Suguro, and S. Masuda, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu, 21st, Hokkaido Daigaku Nogakubu, Sapporo, Japan (1978), p. 370–7; Chem. Abstr., 90, 86689z (1979).
V. S. Joshi, N. P. Damodaran, and S. Dev, Tetrahedron, 27, 459 (1971).
V. K. Agarwal, V. K. Sethi, R. K. Thappa, S. G. Agarwal, and K. L. Dhar, Indian J. Chem., Sect. B, 23, 996 (1984).
R. Appel and H.-D. Wihler, Chem. Ber., 109, 3446 (1976).
G. Yu. Ishmuratov, R. Ya. Kharisov, M. P. Yakovleva, L. P. Botsman, R. R. Muslukhov, and G. A. Tolstikov, Izv. Akad. Nauk, Ser. Khim., 198 (1999).
V. N. Odinokov, G. Yu. Ishmuratov, M. P. Yakovleva, R. L. Safiullin, A. N. Volgarev, V. D. Komissarov, R. R. Muslukhov, and G. A. Tolstikov, Dokl. Akad. Nauk, 326, 842 (1992).
R. V. Stevens, K. T. Chapman, C. A. Stubbs, W. W. Tam, and K. F. Albizati, Tetrahedron Lett., 23, 4647 (1982).
V. N. Odinokov, V. R. Akhmetova, G. Yu. Ishmuratov, L. P. Botsman, and G. A. Tolstikov, Zh. Org. Khim., 22, 953 (1986).
T. Suga, T. Shishibori, and T. Matsuura, J. Org. Chem., 32, 965 (1967).
W. Huckel and C.-M. Jennewein, Liebigs Ann. Chem., 683, 100 (1965).
USSR Pat. No. 1057488, Appl. 24.08.82, No. 34866123-04; Byull. Izobret., No. 4 (1983).
Author information
Authors and Affiliations
Additional information
__________
Translated from Khimiya Prirodnykh Soedinenii, No. 1, pp. 33–36, January–February, 2005.
Rights and permissions
About this article
Cite this article
Ishmuratov, G.Y., Yakovleva, M.P., Ganieva, V.A. et al. Synthesis of the Promising Chiral Synthon Isopropyl-4R-Methyl-6-Iodohexanoate from L-(-)-Menthol. Chem Nat Compd 41, 41–44 (2005). https://doi.org/10.1007/s10600-005-0070-6
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10600-005-0070-6