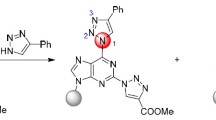
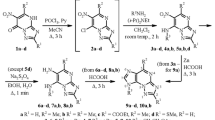
A series of novel compounds containing triazole and phosphonate moieties were obtained under mild conditions. Reactions of 2,6-bis-(triazolyl)purine acyclic nucleoside phosphonates, in which triazole ring at C-6 atom of purine was acting as a good leaving group, and N- or S-nucleophiles allowed to obtain the respective 2-triazolylpurine phosphonates in 62–87% yields.


Similar content being viewed by others
References
(a) De Clercq, E.; Holý, A. Nat. Rev. Drug Discovery 2005, 4, 928. (b) Holý, A. Antiviral Res. 2006, 71, 248. (c) De Clercq, E. Biochem. Pharmacol. 2011, 82, 99.
(a) Andrei, G.; Topalis, D.; De Schutter, T.; Snoeck, R. Antiviral Res. 2015, 114, 21. (b) Lin, C.-L.; Yang, H.-C.; Kao, J.-H. Liver Int. 2016, 36, 85.
Macchi, B.; Romeo, G.; Chiacchio, U.; Frezza, C.; Giofrè, S. V.; Marino-Merlo, F.; Mastino, A. Phosphonated Nucleoside Analogues as Antiviral Agents; Springer: Berlin, 2013, Vol. 10, p. 53.
(a) Klejch, T.; Pohl, R.; Janeba, Z.; Sun, M.; Keough, D. T.; Guddat, L. W.; Hocková, D. Tetrahedron 2018, 74, 5886. (b) Klejch, T.; Keough, D. T.; Chavchich, M.; Travis, J.; Skácel, J.; Pohl, R.; Janeba, Z.; Edstein, M. D.; Avery, V. M.; Guddat, L. W.; Hocková, D. Eur. J. Med. Chem. 2019, 183, 111667. (c) Keough, D. T.; Hockova, D.; Janeba, Z.; Wang, T.-H.; Naesens, L.; Edstein, M. D. J. Med. Chem. 2015, 58, 827. (d) Cheviet, T.; Wein, S.; Bourchenin, G.; Lagacherie, M.; Périgaud, C.; Cerdan, R.; Peyrottes, S. J. Med. Chem. 2020, 63, 8069. d Keough, D. T.; Hocková, D.; Holý, A.; Naesens, L. M. J.; Skinner-Adams, T. S.; de Jersey, J.; Guddat, L. W. J. Med. Chem. 2009, 52, 4391. e de Jersey, J.; Holý, A.; Hockova, D.; Naesens, L.; Keough, D. T.; Guddat, L. W. Curr. Top. Med. Chem. 2011, 11, 2085.
Břehová, P.; Šmídková, M.; Skácel, J.; Dračínský, M.; Mertlíková-Kaiserová, H.; Velasquez, M. P. S.; Watts, V. J.; Janeba, Z. ChemMedChem 2016, 11, 2534.
(a) Pomeisl, K.; Beier, P.; Pohl, R.; Krečmerová, M. ChemistrySelect 2016, 1, 2102. (b) Pomeisl, K.; Krečmerová, M.; Pohl, R.; Snoeck, R.; Andrei, G. Tetrahedron 2019, 75, 130529. (c) Kim, C. U.; Luh, B. Y.; Misco, P. F.; Bronson, J. J.; Hitchcock, M. J. M.; Ghazzouli, I.; Martin, J. C. J. Med. Chem. 1990, 33, 1207.
Brel, V. K. Synthesis 2012, 2359.
(a) Hocková, D.; Janeba, Z.; Naesens, L.; Edstein, M. D.; Chavchich, M.; Keough, D. T.; Guddat, L. W. Bioorg. Med. Chem. 2015, 23, 5502. (b) Hocková, D.; Rosenbergová, Š.; Ménová, P.; Páv, O.; Pohl, R.; Novák, P.; Rosenberg, I. Org. Biomol. Chem. 2015, 13(15), 4449. (c) Głowacka, I. E.; Piotrowska, D. G.; Andrei, G.; Schols, D.; Snoeck, R.; Wróblewski, A. E. Monatsh. Chem. 2016, 147, 2163.
Sahu, P. K.; Kim, G.; Nayak, A.; Ahn, J. Y.; Ha, M. W.; Park, C.; Yu, J.; Park, H.-G.; Jeong, L. S. Asian J. Org. Chem. 2016, 5, 183.
Malnuit, V.; Smoleń, S.; Tichý, M.; Poštová Slavětínská, L.; Hocek, M. Eur. J. Org. Chem. 2019, 5409.
(a) Baszczyňski, O.; Jansa, P.; Dračínský, M.; Klepetářová, B.; Holý, A.; Votruba, I.; de Clercq, E.; Balzarini, J.; Janeba, Z. Bioorg. Med. Chem. 2011, 19, 2114. (b) Manvar, A.; Shah, A. Tetrahedron 2013, 69, 680. (c) Fu, X. Z.; Ou, Y.; Xin, J.; Yang, Y. S. Chin. Chem. Lett. 2011, 22, 1387.
(a) Novosjolova, I.; Bizdēna, Ē.; Turks, M. Eur. J. Org. Chem. 2015, 3629. (b) Jovaisaite, J.; Cīrule, D.; Jeminejs, A.; Novosjolova, I.; Turks, M.; Baronas, P.; Komskis, R.; Tumkevicius, S.; Jonusauskas, G.; Jursenas, S. Phys. Chem. Chem. Phys. 2020, 22, 26502.
(a) Kovaļovs, A.; Novosjolova, I.; Bizdēna, Ē.; Bižāne, I.; Skardziute, L.; Kazlauskas, K.; Jursenas, S.; Turks, M. Tetrahedron Lett. 2013, 54, 850. (b) Novosjolova, I.; Bizdēna, Ē.; Turks, M. Tetrahedron Lett. 2013, 54, 6557. (c) Novosjolova, I.; Bizdēna, Ē.; Turks, M. Phosphorus, Sulfur Silicon Relat. Elem. 2015, 190, 1236.
(a) Lomoschitz, C. J.; Feichtenschlager, B.; Moszner, N.; Puchberger, M.; Müller, K.; Abele, M.; Kickelbick, G. Langmuir 2011, 27, 3534. (b) Cristau, H. J.; Virieux, D. Tetrahedron Lett. 1999, 40, 703.
Ozols, K.; Cīrule, D.; Novosjolova, I.; Stepanovs, D.; Liepinsh, E.; Bizdēna, Ē.; Turks, M. Tetrahedron Lett. 2016, 57, 1174.
Houghton, S. R.; Melton, J.; Fortunak, J.; Brown Ripin, D. H.; Boddy, C. N. Tetrahedron 2010, 66, 8137.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2021, 57(1), 55–62
Supplementary Information
ESM 1
(PDF 3255 kb)
Rights and permissions
About this article
Cite this article
Kapilinskis, Z., Novosjolova, I., Bizdēna, Ē. et al. Synthesis of 2-triazolylpurine Phosphonates. Chem Heterocycl Comp 57, 55–62 (2021). https://doi.org/10.1007/s10593-021-02867-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-021-02867-w