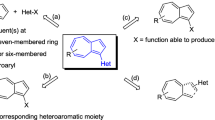
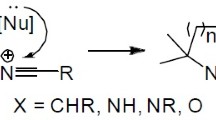
The review summarizes the data for the 2005–2020 period on the methods of synthesis of heterocycles containing the 3,3-dicyanoacrylamide fragment. A group of reactions based on the introduction of the ylidenemalononitrile fragment into an existing 2-oxo-containing azaheterocycle is considered. A number of processes have been described in which simultaneous formation of both the azaheterocycle and the 3,3-dicyanoacrylamide fragment takes place.











Similar content being viewed by others
References
Deng, G.; Wang, Q.; Yang, M.; Li, B.; Han, T.; Chang, B.; Li, X.; Zhang, X.; Li, Z. Mini-Rev. Org. Chem. 2019, 16, 208.
Li, M.; Li, Y.; Zhang, H.; Wang, S.; Ao, Y.; Cui, Z. J. Mater. Chem. C 2017, 5, 4111.
Cho, M. J.; Choi, D. H.; Sullivan, P. A.; Akelaitis, A. J. P.; Dalton, L. R. Prog. Polym. Sci. 2008, 33, 1013.
Sharipova, S. M.; Kalinin, A. A. Chem. Heterocycl. Compd. 2017, 53, 36. [Khim. Geterotsikl. Soedin. 2017, 53, 36.]
Redon, S.; Eucat, G.; Ipuy, M.; Jeanneau, E.; Gautier-Luneau, I.; Ibanez, A.; Andraud, C.; Bretonnière, Y. Dyes Pigm. 2018, 156, 116.
Belikov, M. Yu.; Ievlev, M. Yu.; Fedoseev, S. V.; Ershov, O. V. New J. Chem. 2019, 43, 8414.
Reig, M.; Puigdollers, J.; Velasco, D. Phys. Chem. Chem. Phys. 2018, 20, 1142.
Al Garni, S. E.; Darwish, A. A. A. Phys. Scr. 2020, 95, ID 045806. https://doi.org/10.1088/1402-4896/ab623e.
King, J. A., Jr.; Houser, C. L.; Corkill, R. E.; Yee, G. T. J. Magn. Magn. Mater. 2020, 497, 165953.
Podlesný, J.; Pytela, O.; Klikar, M.; Jelínková, V.; Kityk, I. V.; Ozga, K.; Jedryka, J.; Rudyshd, M.; Bureš, F. Org. Biomol. Chem. 2019, 17, 3623.
Castro, M. C. R.; Belsley, M.; Raposo, M. M. M. Dyes Pigm. 2016, 131, 333.
Zhao, D.; Hu, J.; Liu, Z.; Xiao, B.; Wang, X.; Zhou, E.; Zhang, Q. Dyes Pigm. 2018, 151, 102.
Zhao, D.; Hu, J.; Cao, K.; Xiao, B.; Zhou, E.; Zhang, Q. Dyes Pigm. 2019, 162, 898.
Dhondge, A. P.; Chen, J.-Y.; Lin, T.; Yen, F.-M.; Li, K.-W.; Hsieh, H.-C.; Kuo, M.-Y. Org. Lett. 2018, 20, 40.
Dhondge, A. P.; Tsai, P.-C.; Nien, C.-Y.; Xu, W.-Y.; Chen, P.-M.; Hsu, Y.-H.; Li, K.-W.; Yen, F.-M.; Tseng, S.-L.; Chang, Y.-C.; Chen, H. J. H.; Kuo, M.-Y. Org. Lett. 2018, 20, 2538.
Dhondge, A. P.; Huang, Y.-X.; Lin, T.; Hsu, Y.-H.; Tseng, S.-L.; Chang, Y.-C.; Chen, H. J. H.; Kuo, M.-Y. J. Org. Chem. 2019, 84, 14061.
Chen, R.; Zhang, G.; Zhou, W. CN Patent 110790776, 2020.
Tafeenko, V. A.; Gurskiy, S. I. Cryst. Growth Des. 2016, 16, 940.
El-Sharief, A. M. Sh.; Ammar, Y. A.; Belal, A.; El-Sharief, M. A. M. Sh.; Mohamed, Y. A.; Mehany, A. B. M.; Elhag Ali, G. A. M.; Ragab, A. Bioorg. Chem. 2019, 85, 399.
Salem, M. A.; Ragab, A.; Askar, A. A.; El-Khalafawy, A.; Makhlouf, A. H. Eur. J. Med. Chem. 2020, 188, 111977.
El-Gaby, M. S. A.; El-Hag Ali, G. A. M.; El-Maghraby, A. A.; Abd El-Rahman, M. T.; Helal, M. H. M. Eur. J. Med. Chem. 2009, 44, 4148.
Chu, W.; Rothfuss, J.; d’Avignon, A.; Zeng, C.; Zhou, D.; Hotchkiss, R. S.; Mach, R. H. J. Med. Chem. 2007, 50, 3751.
Chu, W.; Rothfuss, J.; Zhou, D.; Mach, R. H. Bioorg. Med. Chem. Lett. 2011, 21, 2192.
Gupta, N.; Roy, T.; Ghosh, D.; Abdi, S. H. R.; Kureshy, R. I.; Khan, N. H.; Bajaj, H. C. RSC Adv. 2015, 5, 17843.
Zaghari, Z.; Azizian, J. Comb. Chem. High Throughput Screening 2018, 21, 609.
Demchuk, D. V.; Elinson, M. N.; Nikishin, G. I. Mendeleev Commun. 2011, 21, 224.
Tan, Z.-Y.; Wu, K.-X.; Huang, L.-S.; Wu, R.-S.; Du, Z.-Y.; Xu, D.-Z. Green Chem. 2020, 22, 332.
Huang, L.-S.; Lai, Y.-H.; Yang, C.; Xu, D.-Z. Appl. Organomet. Chem. 2019, 33, E4910.
Androsov, D. A.; Kishbaugh, T. L. S.; Gribble, G. W. Tetrahedron Lett. 2008, 49, 6621.
Ghozlan, S. A. S.; Ramadan, M. A.; Abdelmoniem, A. M.; Elwahy, A. H. M.; Abdelhamid, I. A. Turk. J. Chem. 2017, 41, 410.
Wu, S.-X.; Gu, B.-Q.; Xu, H.; Zheng, X.; Luo, X.; Deng, W.-P. Adv. Synth. Catal. 2019, 361, 4302.
Sabitov, A. A.; Dmitriev, M. V.; Belozerova, A. I.; Sal’nikova, T. V.; Maslivets, A. N. Russ. J. Org. Chem. 2020, 56, 1217. [Zh. Org. Khim. 2020, 56, 1109.]
Sal’nikova, T. V.; Dmitriev, M. V.; Bushmeleva, E. V.; Silaichev, P. S.; Maslivets, A. N. Russ. J. Org. Chem. 2018, 54, 564. [Zh. Org. Khim. 2018, 54, 564.]
Dmitriev, M. V.; Silaichev, P. S.; Maslivets, A. N. Russ. J. Org. Chem. 2015, 51, 74. [Zh. Org. Khim. 2015, 51, 77.]
Milovidova, A. G.; Belikov, M. Yu.; Ievlev, M. Yu.; Ershov, O. V.; Nasakin, O. E. Russ. J. Org. Chem. 2018, 54, 1790. [Zh. Org. Khim. 2018, 54, 1776.]
Youssef, M. M.; Amin, M. A. Molecules 2010, 15, 8827.
Faty, R. M.; Rashed, M. S.; Youssef, M. M. Molecules 2015, 20, 1842.
Aly, M. M. Phosphorus, Sulfur Silicon Relat. Elem. 2007, 182, 1497.
Burgess, J.; Steel, P. J. Tetrahedron Lett. 2006, 47, 4107.
Belikov, M. Yu.; Ievlev, M. Yu.; Milovidova, A. G.; Ershov, O. V. Russ. J. Org. Chem. 2017, 53, 1601. [Zh. Org. Khim. 2017, 53, 1565.]
Milovidova, A. G.; Belikov, M. Yu.; Ievlev, M. Yu.; Ershov, O. V.; Nasakin, O. E. Tafeenko, V. A. Tetrahedron Lett. 2020, 61, 151368.
Khil, A. M.; Kaminskii, V. A.; Slabko, O. Yu.; Kachanov, A. V.; Gerasimenko, A. V. J. Heterocycl. Chem. 2015, 52, 688.
Kayukova, O. V.; Kayukov, Ya. S.; Nikolaev, A. N.; Tafeenko, V. A.; Ershov, O. V.; Nasakin, O. E. Russ. J. Org. Chem. 2005, 41, 523. [Zh. Org. Khim. 2005, 41, 535.]
Kayukov, Ya. S.; Kayukova O. V.; Kalyagina, E. S.; Bardasov, I. N.; Ershov, O. V.; Nasakin, O. E.; Tafeenko, V. A. Russ. J. Org. Chem. 2011, 47, 392. [Zh. Org. Khim. 2011, 47, 400.]
Ershov, O. V.; Ievlev, M. Yu. Chem. Heterocycl. Compd. 2017, 53, 948. [Khim. Geterotsikl. Soedin. 2017, 53, 948.]
This work was financially supported by the Russian Foundation for Basic Research (project No. 18-33-20268 mol_a_ved).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2021, 57(1), 1–6
Rights and permissions
About this article
Cite this article
Belikov, M.Y., Milovidova, A.G. Synthesis of heterocyclic compounds containing the 3,3-dicyanoacrylamide fragment. Chem Heterocycl Comp 57, 1–6 (2021). https://doi.org/10.1007/s10593-021-02858-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-021-02858-x