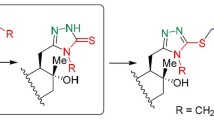
Synthesis of novel (+)-sclareolide-based homodrimane sesquiterpenoids bearing 1,3,4-oxadiazole or 1,3,4-thiadiazole units has been reported. These terpeno-heterocyclic compounds demonstrate promising antifungal and antibacterial activities toward fungal species Aspergillus niger, Fusarium solani, Penicillium chrysogenum, Penicillium frequentans, and Alternaria alternata and bacteria strains Pseudomonas aeruginosa and Bacillus sp. with minimum inhibitory concentration at μg/ml level.


Similar content being viewed by others
References
Grahman, P. L. An Introduction to Medicinal Chemistry; Oxford University Press: New York, 2001, 2nd ed.
Greenwood, D.; Finch, R.; Davey, P.; Wilcox, M. Antimicrobial Chemotherapy; Oxford University Press: New York, 2007, 5th ed.
Fraga, B. M. Nat. Prod. Rep.2013, 30, 1226.
Jansen, B. J. M.; de Groot, A. Nat. Prod. Rep.2004, 21, 449.
Patel, N. B.; Patel, J. C. Sci. Pharm.2010, 78, 171.
Xu, J.; Wang, D.-L.; Imafuku, K. Synth. Commun.2009, 39, 2196.
Kashaw, S. K.; Gupta, V.; Kashaw, V.; Mishra, P.; Stables, J. P.; Jain, N. K. Med. Chem. Res.2010, 19, 250.
Bondock, S.; Adel, S.; Etman, H. A.; Badria, F. A. Eur. J. Med. Chem.2012, 48, 192.
Salgın-Gökşen, U.; Gökhan-Kelekçi, N.; Göktaş, Ö.; Köysal, Y.; Kılıç, E.; Işık, Ş.; Aktay, G.; Özalp, M. Bioorg. Med. Chem. 2007, 15, 5738.
Hu, Y.; Li, C.-Y.; Wang, X.-M.; Yang, Y.-H.; Zhu, H.-L. Chem. Rev.2014, 114, 5572.
Soares de Oliveira, C.; Lira, B. F.; Barbosa-Filho, J. M.; Lorenzo, J. G. F.; Filgueiras de Athayde-Filho, P. Molecules2012, 17, 10192.
Kuchkova, K.; Aricu, A.; Secara. E.; Barba, A.; Vlad, P.; Ungur, N.; Tuchilus, C.; Shova, S.; Zbancioc, G.; Mangalagiu, I. I. Med. Chem. Res.2014, 23, 1559.
Kuchkova, K. I.; Arycu, A. N.; Sekara, E. S.; Barba, A. N.; Vlad, P. F.; Makaev, F. Z.; Mel'nik, E.; Kravtsov, V. K. Chem. Nat. Compd.2015, 51, 684. [Khim. Prir. Soedin.2015, 589.]
Aricu, A.; Ciocarlan, A.; Lungu, L.; Barba, A.; Shova, S.; Zbancioc, G.; Mangalagiu, I. I.; D’Ambrosio, M.; Vornicu, N. Med. Chem. Res.2016, 25, 2316.
Styngach, E. P.; Malinovskii, S. T.; Bets, L. P.; Vlad, L. A.; Gdanets, M.; Makaev, F. Z. J. Struct. Chem.2005, 46, 765.
Rusu, G. G.; Gutu, E. E.; Barba, N. A. Russ. J. Org. Chem.1995, 31, 1721. [Zh. Org. Khim.1995, 31, 1721.]
Boi, L. V. Russ. Chem. Bull., Int. Ed.2000, 49, 335. [Izv. Akad. Nauk, Ser. Khim.2000, 334.]
Linganna, N.; Lokanatha Rai, K. M. Synth. Commun.1998, 28, 4611.
Boschelli, D. H.; Connor, D. T.; Bornemeier, D. A.; Dyer, R. D.; Kennedy, J. A.; Kuipers, P. J.; Okonkwo, G. C.; Schrier, D. J.; Wright, C. D. J. Med. Chem. 1993, 36, 1802.
Macaev, F.; Rusu, G.; Pogrebnoi, S.; Gudima, A.; Stingaci, E.; Vlad, L.; Shvets, N.; Kandemirli, F.; Dimoglo, A.; Reynolds, R. Bioorg. Med. Chem.2005, 13, 4842.
Aryanasab, F.; Halimehjani, A. Z.; Saidi, M. R. Tetrahedron Lett. 2010, 51, 790.
Lungu, L.; Ciocarlan, A.; Barba, A.; Shova, S.; Pogrebnoi, S.; Mangalagiu, I.; Moldoveanu, C.; Vornicu, N.; D’Ambrosio, M.; Babak, M. V.; Arion, V. B.; Aricu, A. Chem. Heterocycl. Compd.2019, 55, 716. [Khim. Geterotsikl. Soedin.2019, 55, 716.]
Omar, F. A.; Mahfouz, N. M.; Rahman, M. A. Eur. J. Med. Chem.1996, 31, 819.
Antimicrobial Susceptibility Testing (AST) Standards M07 and M100; National Committee on Clinical Laboratory Standards: Wayne, 2003.
CrysAlis RED, v1.171.38.46; Oxford Diffraction Ltd.: Abingdon, 2003.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr.2009, 42, 339.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem.2015, C71, 3.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(5), 578–585
Electronic supplementary material
ESM1
(PDF 3403 kb)
Rights and permissions
About this article
Cite this article
Lungu, L., Ciocarlan, A., Smigon, C. et al. Synthesis and Evaluation of Biological Activity of Homodrimane Sesquiterpenoids Bearing 1,3,4-Oxadiazole or 1,3,4-Thiadiazole Units. Chem Heterocycl Comp 56, 578–585 (2020). https://doi.org/10.1007/s10593-020-02703-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02703-7