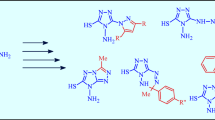
Alkylation of 4,6-dimethyl-2-sulfanylpyridine-3-carbonitrile with 1-aryl-5-(chloromethyl)-1H-tetrazoles yielded 2-{[(1-aryl-1H-tetrazol-5-yl)methyl]sulfanyl}-4,6-dimethylpyridine-3-carbonitriles, which easily cyclize by the action of bases to form 2-(1H-tetrazol-5-yl)-thieno[2,3-b]pyridine derivatives. The use of excess base in the alkylation step leads to direct formation of cyclized products in high yields.

Similar content being viewed by others
References
(а) Litvinov, V. P.; Dotsenko, V. V.; Krivokolysko, S. G. Chemistry of Thienopyridines and Related Systems [in Russian]; Belen'kii, L. I., Ed.; Nauka: Moscow, 2006, p. 5. (b) Salem, M. E.; Darweesh, A. F.; Elwahy, A. H. M. J. Sulfur Chem. 2018, 39, 525. (c) Dyachenko, I. V.; Dyachenko, V. D.; Dorovatovskii, P. V.; Khrustalev, V. N.; Nenajdenko, V. G. Russ. J. Org. Chem. 2018, 54, 1435. [Zh. Org. Khim. 2018, 54, 1423.]
(a) Rolim Bernardino, A. M.; da Silva Pinheiro, L. C.; Rodrigues, C. R.; Loureiro, N. I.; Castro, H. C.; Lanfredi-Rangel, A.; Sabatini-Lopes, J.; Borges, J. C.; Carvalho, J. M.; Romeiro, G. A.; Ferreira, V. F.; Frugulhetti, I. C. P. P.; Vannier-Santos, M. A. Bioorg. Med. Chem. 2006, 14, 5765. (b) Al-Trawneh, S. A.; El-Abadelah, M. M.; Zahra, J. A.; Al-Taweel, S. A.; Zani, F.; Incerti, M.; Cavazzoni, A.; Vicini, P. Bioorg. Med. Chem. 2011, 19, 2541.
Chaubey, A.; Pandeya, S. N. Asian J. Pharm. Clin. Res. 2011, 4, 5.
(a) Madhusudana, K.; Shireesha, B.; Modi Naidu, V. G.; Ramakrishna, S.; Narsaiah, B.; Rao, A. R.; Diwan, P. V. Eur.J. Pharmacol. 2012, 678, 48. (b) Liu, H.; Li, Y.; Wang, X.-Y.; Wang, B.; He, H.-Y.; Liu, J.-Y.; Xiang, M.-L.; He, J.; Wu, X.-H.; Yang, L. Bioorg. Med. Chem. Lett. 2013, 23, 2349.
(а) Bahekar, R. H.; Jain, M. R.; Goel, A.; Patel, D. N.; Prajapati, V. M.; Gupta, A. A.; Javad, P. A.; Patel, P. R.Bioorg. Med. Chem. 2007, 15, 3248. (b) Kamata, M.; Yamashita, T.; Kina, A.; Funata, M.; Mizukami, A.; Sasaki, M.; Tani, A.; Funami, M.; Amano, N.; Fukatsu, K. Bioorg. Med. Chem. Lett. 2012, 22, 3643.
Adachi, I.; Yamamori, T.; Hiramatsu, Y.; Sakai, K.; Mihara, S.; Kawakami, M.; Masui, M.; Uno, O.; Ueda, M. Chem. Pharm.Bull. 1988, 36, 4389.
Pevet, I.; Brulé, C.; Tizot, A.; Gohier, A.; Cruzalegui, F.; Boutin, J. A.; Goldstein, S. Bioorg. Med. Chem. 2011, 19, 2517.
Herr, R. J. Bioorg. Med. Chem. 2002, 10, 3379.
Abdel-Rahman, A. E.; Bakhite, E. A.; Mohamed, O. S.; Thabet, E. A. Phosphorus, Sulfur Silicon Relat. Elem. 2003, 178, 89.
(a) Gewald, K.; Hentschel, M.; Illgen, U. J. Prakt. Chem. 1974, 316, 1030. (b) Ivanov, V. L.; Artemov, V. A.; Shestopalov, A. M.; Litvinov, V. P. Chem. Heterocycl. Compd. 1998, 34, 237. [Khim. Geterotsikl. Soedin. 1998, 263.] (c) Sherman, A. R. In Comprehensive Heterocyclic Chemistry III; Katritzky, A. R.; Scriven, E. F. V.; Ramsden, C. A.; Taylor, R. J. K., Eds.; Elsevier: New York, 2008, Vol. 10, p. 263.
Babichev, F. S.; Sharanin, Yu. A.; Promonenkov, V. K.; Litvinov, V. P.; Volovenko, Yu. M. Intramolecular Interaction of Nitrile Group with С-Н, О-Н, and S-H Groups [in Russian]; Babichev, F. S., Ed.; Naukova dumka: Kiev, 1985, p. 33.
Narushyavichus, É. V.; Garalene, V. N; Krauze, A. A.; Dubur, G. Ya. Pharm. Chem. J. 1989, 23, 983. [Khim. Farm. Zh. 1989, 23, 1459.]
Kochetkov, N. K.; Khomutova, E. D.; Bazilevskii, M. V. Zh. Org. Khim. 1958, 28, 2736.
Harvill, E. K.; Herbst, R. M.; Schreiner, E. G. J. Org. Chem. 1952, 17, 1597.
Fizer, L.; Fizer, M. Reagents for Organic Synthesis [Russian translation]; Knunyants, I. L.; Kostyanovsky, R. G., Eds.; Mir: Moscow, 1970, Vol. 1, p. 25.
Cosgrove, C. E.; La Forge, R. A. J. Org. Chem. 1956, 21, 197.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(8), 768–772
Rights and permissions
About this article
Cite this article
Koltsov, N.Y. Synthesis of 2-(1-aryl-1H-tetrazol-5-yl)-thieno[2,3-b]pyridine derivatives. Chem Heterocycl Comp 55, 768–772 (2019). https://doi.org/10.1007/s10593-019-02533-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02533-2